Auriculares and the Sonus Feather
A detailed study of bird auriculares
Species count: 53
Orders: 14
Measurements: 795
Plucked feathers: 3952
Feather Series
- A Review of Nomenclature for Avian Color Aberrations
- Feather Basics
- There Are Nine Types of Feathers
- Morphology and Morphometric Characterization of Barn Owl External Sound Related Adaptations
- Auriculares and the Sonus Feather
site under construction with disjointed sections
- note that interpretations have not been posted to species level
- this page being updated as research progresses
- first sections published 3/11/24;
- dust protector 3/16/24
- types of auricular feathers 4/1/24
- sonus afterfeather 4/2/24
- meatus 4/3/24
- meatus scaling 4/4/24
- not just auriculares 4/29/24
- research details by order 5/13/24
- study species updated, details posted to each species page, no interpretation 5/22/24
- calamus 7/7/24
- White-breasted Nuthatch, species #53 8/9/24
- Bald Eagle study — I succeed in getting access permission to study a Bald Eagle. Timeline is based on availability. This is a watershed moment for the auricular study. 8/30/24
- current initiatives — evolution and the Palaeognathae (ostrich, kiwi, tinamou), update to the Barn Owl article
Auricular Definition
Auricular feathers cover the bird’s ear which is inferior and posterior to the eye. These feathers are also called ear coverts or an ear patch. Currently auriculares are a subset of contour feathers. Auriculares are not usually noticeable but occasionally a bird will raise them.

Inca Dove auriculares

White-winged Dove auriculares
Types of Auricular Feathers
To date the research has found four types of auricular feathers. All birds in the study group except the Ostrich, have sonus feathers (author’s term) of various morphologies. The Ostrich has bristle feathers for ear coverts and the American Kestrel has some bristles amongst its sonus feathers. The Costa’s Hummingbird has some contour feathers covering its meatus. Finally, all the sources agree that there are filoplumes associated with all feathers. The sonus is no exception.
From left to right, an Ostrich bristle feather, a Barn Owl sonus feather, a Costa’s Hummingbird contour feather, and the far right, a Peregrine Falcon meatus showing filoplumes.
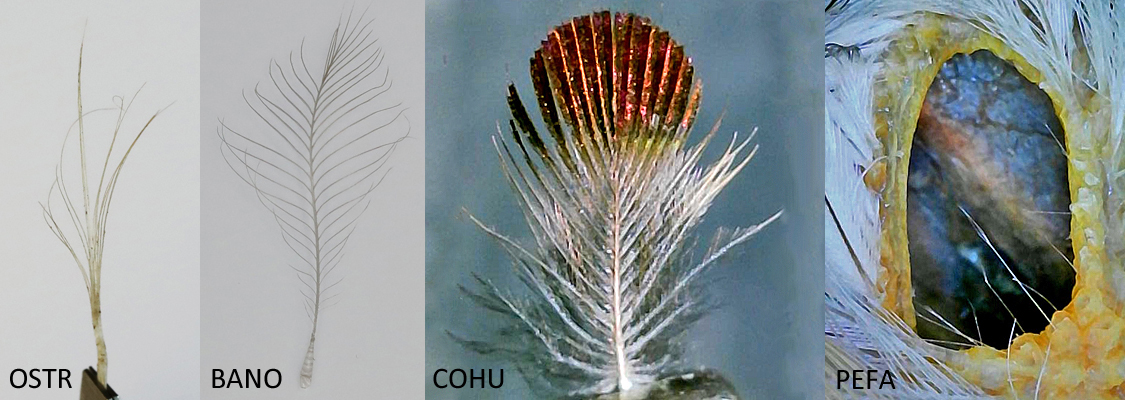
auricular feather types
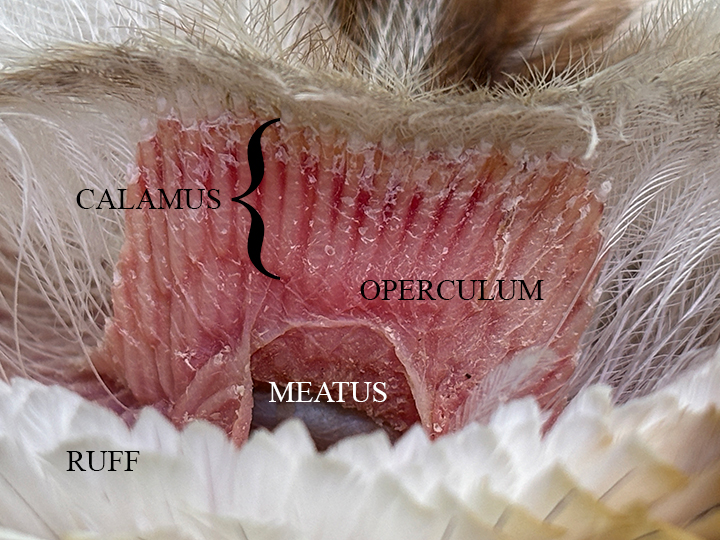
Meatus
The meatus is the external opening to the ear canal. It is situated slightly behind and below the eye. All of the birds in the study, with the exception of the Barn Owl, have auricular feathers covering the meatus. The Barn Owl has a pre-aural flap, called an operculum, covering the meatus which itself is covered by sonus feathers. The meatus of the Ostrich (OSTR) and the Ring-necked Pheasant (RNEP) are covered by different types of auricular feathers. In both cases, although not discernible in the picture for the RNEP, the meatus can be seen through the auriculares.
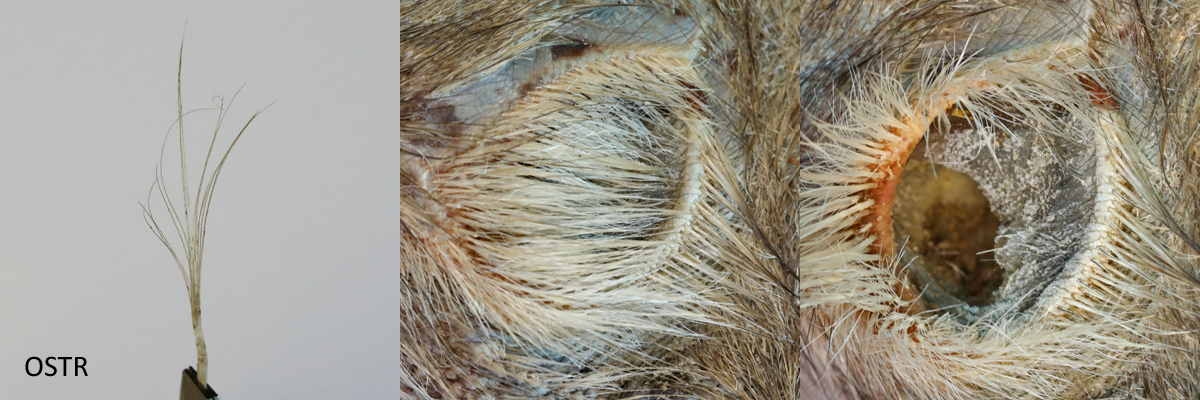
Ostrich bristle auriculares and meatus
Notice in the RNEP series that the sonus feather is fully splayed in the first frame. This is due to static electricity. When covering the meatus they lay compact resulting in greater coverage. Once the sonus is plucked, measuring the width would not be a valid measurement.
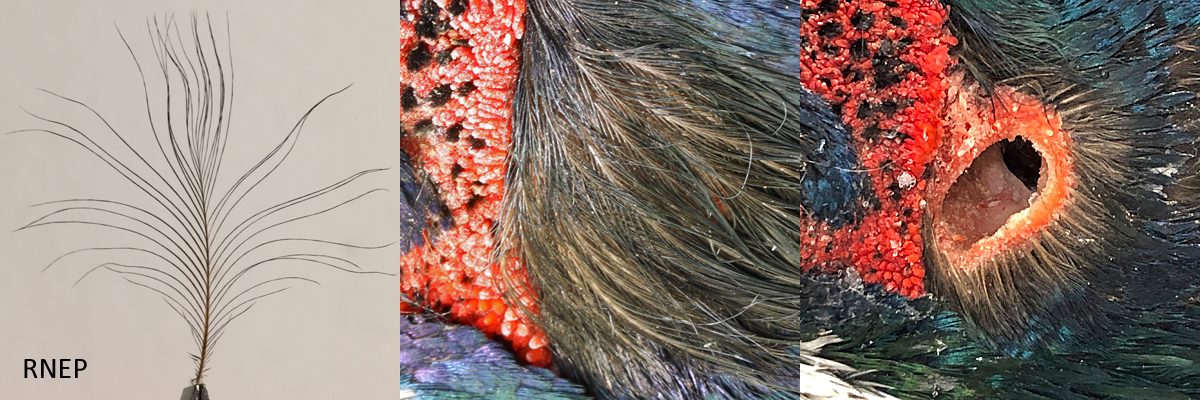
Ring-necked Pheasant sonus auricular and meatus
The Barn Owl (BANO) has the only bare meatus in the study group. It is bordered by the ruff, operculum and specialized sonus feathers. The Greater Roadrunner (GRRO) has the largest meatus in relation to its skull size in the study group.

Barn Owl and Greater Roadrunner meatus
The Dark-eyed Junco (DEJU) and Abert’s Towhee exemplify the most common look of the meatus and the surrounding auriculares, sonus in this case.
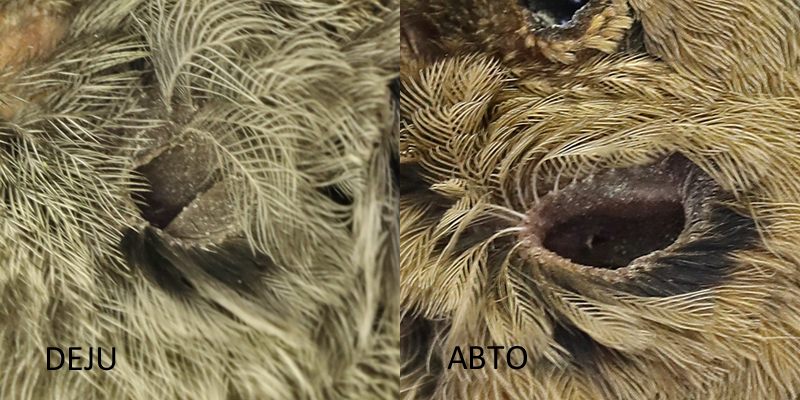
Dark-eyed Junco and Abert’s Towhee meatus
Meatus Scaling
Measurements were made of the meatus to calculate its area and the skull to estimate volume (see methods). A ratio was calculated and plotted. When plotted, the ratios for the Great Horned Owl and the Ostrich caused a loss of fidelity for data on the x-axis. The pictures on the right of the graph estimate where the ratios would plot to the y-axis.
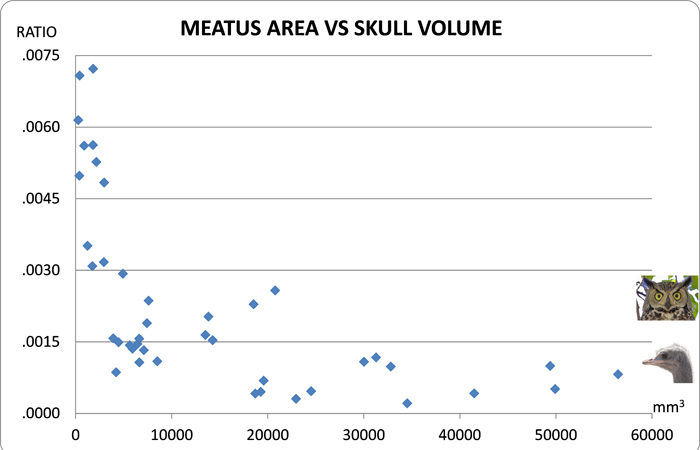
Meatus Area vs Skull Volume
As the skull reduces in size (left end of X-axis) the meatus becomes relatively larger (top end of Y-axis). This indicates that there is a limit to reduction in meatus area as the skull gets smaller. The meatus of a hummingbird is 6 times larger than an ostrich on a relative scale.
An interesting article about estimating t-rex brain size introduced me to the concept of scaling.13 As seen in the above graph, it is difficult to compare a hummingbird to an Ostrich. Comparing an elephant to a mouse would make the x-axis kilometers long.14 To allow for comparisons of Ostriches to hummingbirds a log-transformation chart is used. The same data as the graph above is used for this next graph.
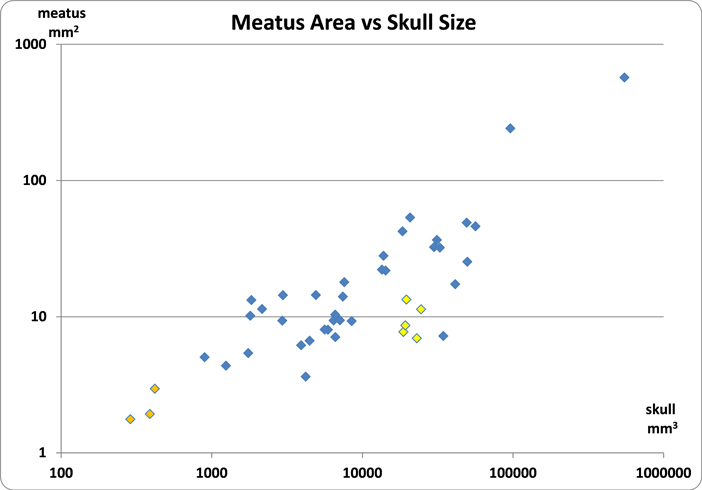
Meatus Area vs Skull Volume
In this graph the orange points are hummingbirds which visibly seem to fit with the Ostrich plotted on the upper right. The data interpretation says that as skulls get larger the meatus area gets proportionately smaller.
The yellow diamonds belong to the five ducks in the study. In comparison to other species with the same skull size, the ducks have a smaller than expected meatus area. This would fit if a smaller meatus helps protect the duck’s ear from water. The two lowest duck points on the graph are diving ducks, further supporting the evolutionary pressure to protect the ear.
The data point below and to the right of the ducks belongs to the chicken. Domestic chickens are raised in tight quarters without predators. Over 70 billion chickens are raised each year compressing evolutionary changes. A smaller meatus would seem a likely outcome.
Calamus
The calamus or quill is the proximal end of a feather that is seated in the follicular cavity below the surface of the skin.

chicken feather calamus
During feather development the pin feather is fed nutrients via blood supplied by the axial artery through the dermal papilla into the pulp.

Mourning Dove dermal papilla and pulp
Once the feather has matured, the dermal papilla is absorbed back into the body and the calamus becomes hollow. This feature allows the storage of ink for a quill pen. The feather at this point is a collection of dead cells, like the tip of your fingernail. Close inspection of the mature calamus shows bands called “pulp caps” as seen on this Barn Owl primary covert.

Barn Owl pulp cap
These pulp cap rings are the remnants of the blood vessels that supplied the feather with nutrients while it was growing.
Using the Barn Owl as the paradigm for optimal hearing, one of its many unusual traits is the exceptionally long calamus on the auriculares and ruff feathers surrounding the meatus.
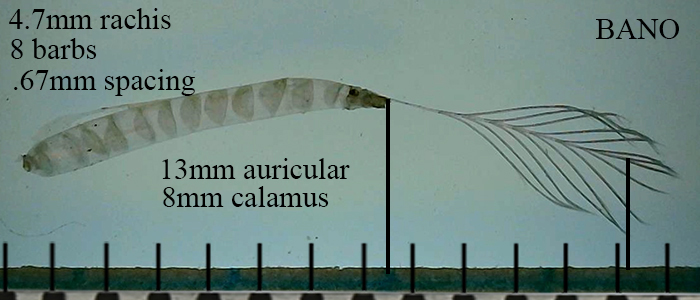
Barn Owl auricular
The calamus serves as the anchor that secures the feather to the bird’s skin. Under the skin, surrounding the calamus, are erector and depressor muscles and ones that control sideways movements of constriction and expansion.
I postulate that, like the baseball bat analogy in following picture, the greater the length of the calamus (using two hands), the greater control of the feather’s position.

base support
The long calamus is an evolutionary adaptation that helps the Barn Owl control its operculum and ruff which are moved independently to isolate the source of the sound. Since control of feathers surrounding the meatus enhances hearing in owls, it would be anticipated that other orders would have this trait.
Review of the orders examined in this research reveals an interesting hierarchy. With the simple rule that a proportionally longer calamus implies control of auriculares and better optimize hearing, ducks have the smallest percentage of calamus to total shaft length at 7% followed by hawks at 8%, passerines at 9%, dove at 17% and ground birds at 18%. As in other traits, ducks sacrifice hearing for waterproofing. Hawks hunt primarily by sight while the hunted, doves and ground birds, use hearing as a defensive tool.
All birds can raise their feathers like the Bald Eagle (Fig. 6). I conjecture that the length of the auricular calamus is related to the bird’s ability to singularly control just the ear coverts as shown by the doves.

BAEA WWDO INDO
Another function of a long calamus is structural. The frame of a hand fan gives rigidity to the surface thus holding its shape.

area support
The meatus of the Long-eared Owl is exceptionally large with each about a third of the circumference of the head. Shaped like a colon (Fig 7), the walls of the meatus are stabilized by feathers with long calami.
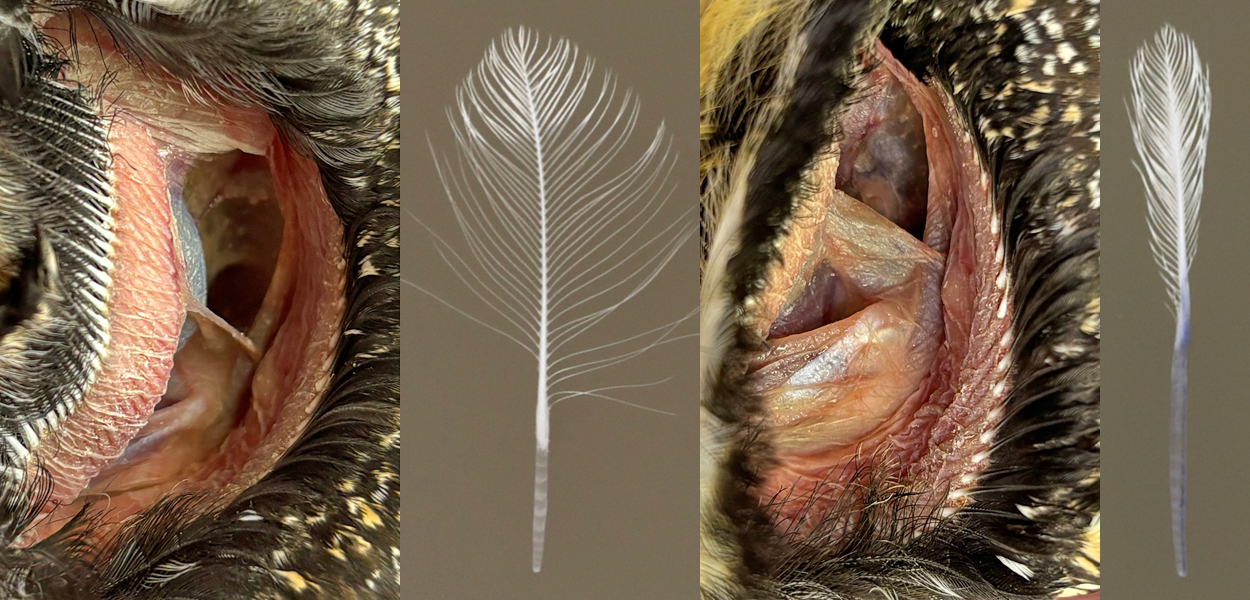
Long-eared Owls LEOW meatus and auriculares showing embedded calami
The Barn Owl uses auricular calami for structural support of its operculum which can be raised or lowered independently.
Barn Owl BANO OPERCULUM
Sonus Feather Morphotypes
Sonus Afterfeather
Afterfeathers (hypopnea) are a secondary feather growing from the dorsal side of a contour feather. It emerges at the base of the vane. The barbs are plumulaceous that sometimes attach to a rachis as in this case. Their appearance is like a downy feather and serves the same purpose. The afterfeather’s rachis was bent away from the contour feather for this photograph.

Peregrine Falcon breast contour feather with afterfeather
Modified afterfeathers are found on some sonus feathers. They do not have the downy attributes of a contour afterfeather but have the same emergence location. The microscopy of a sonus afterfeather pulled from a Peregrine Falcon (PEFA) shows a sonus type structure. The first frame shows the afterfeather rachis emerging from the sonus feather at its proximal end. The other three frames show the proximal, medial, and distal portions of the afterfeather. Note the lack of barbs.

sonus afterfeather
Four orders displayed this morphotype across all the species studied within that order. Piciformes’ Acorn Woodpecker (ACWO) and Gila Woodpecker (GIWO). Falconiformes’ American Kestrel (AMKE) and Peregrine Falcon PEFA. Accipitriformes’ Cooper’s Hawk (COHA), Harris’s Hawk (HASH), Sharp-shinned Hawk (SSHA) and Red-tailed Hawk (RTHA). Anseriformes’ American Wigeon (AMWI), Common Goldeneye (COGO), Green-winged Teal (GWTE), Lesser Scaup (LESC), and Norther Shoveler (NSHO). Conclusions on function and prevalence across these orders have not yet been made.

sonus afterfeathers
Questioning Auricular Attributes
Ear protection, wind dampening, reflecting or directing sound are all attributes given to auricular feathers. In Lederer’s study of bristle feathers he observed “attributes seem to be casually accepted without definitive evidence.”9
Dust Protector
It is puzzling to read that primary purpose of Barn Owl (BANO) auriculares is to protect the ruff from dust.12 The immediate reaction is to question the environment of raptors as being dusty. Two birds that thrive in a dusty environment are the Curve-billed Thrasher (CBTH) and Rufous Hummingbird (RUHU). The CBTH forages for food by sweeping the ground turning over rocks and litter and sometimes pecks into the dirt like a woodpecker. The RUHU harvests bugs and nectar from flowering plants subjecting its face to pollen and dust. A hummingbird will feed over a thousand times per day.
These are the sonus auriculares from the three species.

sonus feathers
The BANO’s feather is open with no discernible barbules while the two species from a dusty environment show growth along the rachis and developed barbules. A close up look follows.

distal microscopy of sonus feather
It is clear that the sonus feathers for the CBTH and RUHU are adapted to account for the dust environment. If the primary function of the BANO sonus feather is dust protection, why not have similar adaptations shown in the other two species. More puzzling is why the sonus feather completely covers the area inside the ruff. A sub optimized dust protector on the cheek of a bird would be better served by an ordinary contour feather if protection is its primary function.
Review of sonus morphotypes indicates there are adaptations to a dusty environment for some species. It shouldn’t be concluded that this is the feather function for all species.
Reflecting or Directing Sound
Wind Dampening
Sensory Feathers, Mechanoreceptors
Filoplume
The filoplume is the third type of feather structure; pennaceous and plumulaceous are the other two. A filoplume is a hair-like feather that has a long bare rachis (shaft) with a small narrow vane at the distal end. The vane consists of one to six short barbs with sparse barbules. They come in various lengths depending on the feather they are supporting.

Ring-necked Pheasant RNEP filoplume
Filoplumes grow from their own innervated follicle.1 They are attached to sensory receptors in the skin that detect air pressure, wind and feather movement. Disturbance of a filoplume enlarged tip creates a vibration that is magnified and transmitted by the long, thin shaft to sensory corpuscles at its base.4
Every bird has at least one filoplume per wing, tail or body feather. Flight feathers have up to 12 filoplumes each.3 When grown at the base of a flight feather they are shielded from direct airflow as they do not protrude from under the coverts. They sense the movement, condition, or integration of the companion flight feather.1

filoplume on flight feather
An experiment was conducted on a Golden Eagle, where a rectrix was clipped above the calamus. This species molts feathers every two to three years. The clipped feather was replaced prior to the next molt cycle indicating that the filoplume sensed the missing feather.1
Some filoplumes in Pelecaniformes, Procellariformes, and Passeriformes visibly project beyond the surrounding contour feathers.4 A review of bird skins was undertaken to find protruding filoplumes extending beyond the contour feathers. 117 North American oscine species (song birds) were found to have this feature. Most occurrences were found on the nape and less frequently on the crown. It is speculated that they detect airflow, feather ruffling, and drag during flight controlling contour feathers that are out of sight and not accessible to preening.2
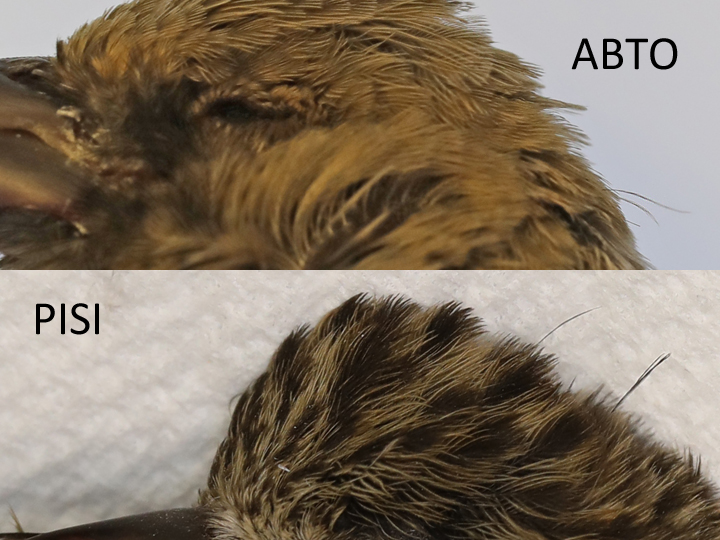
Abert’s Towhee ABTO Pine Siskin PISI nape filoplumes
Bristle feathers
Bristle feathers are found on many species. They are whisker-like feathers typically found around the mouth, eyelids and nares. Bristle feathers maybe unbranched or branched. The branched bristles can have minimum barbs at the base to more elaborate structures as found on nightjars. Some birds have both types of structures as shown in the next picture. It was positively correlated that as bristle numbers increase so too the length.8
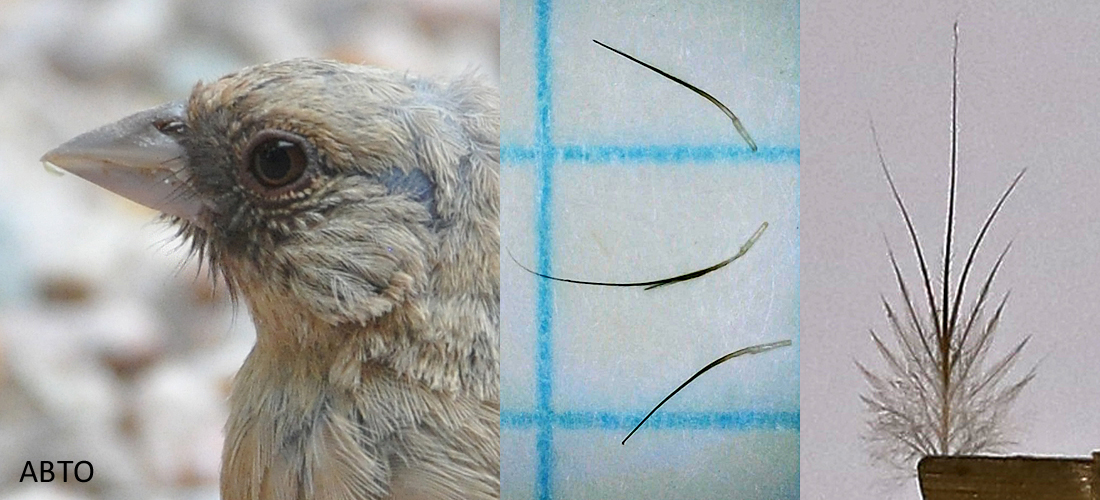
Abert’s Towhee bristles
Bristles are sensitive to touch and vibrations. They assist in foraging, burrowing nests, obstacle avoidance, protection from airborne particles, and sense airflow. Some websites state that bristles are used to funnel food. That was dispelled in an article about Willow Flycatcher bristles.10 Although feathers are dead cells; the bristle feather sits in an innervated follicle connected by muscle tissue within the dermis. The follicle contains Herbst corpuscles, vibration-sensitive mechanoreceptors, that are sensory nerve endings found only in birds.7
A recent study of bristles states that lower and upper rictal bristles were present in the most recent common ancestor (MRCA ~ 108 mya) of avian phylogeny. This feather is diverse with differences in shape, size and position within the same order, family and genera and is sexually monomorphic. In a sample of 1,000 bird species, about a third had bristles. Nocturnality is a predictor of both the presence and length of the bristle feather.6
It was postulated by Küster in 1905 that bristle feathers may be able to sense sound and used in tracking prey.9
click here to see a collection of bristle feathers
Adornment feathers
Whiskered Auklets have three prominent white face plumes and a longer thin crest on its forehead. The crevice dwelling seabird that is active at night was put through a series of experiments involving a totally dark maze. The experiments indicated the elongated facial feather adornments have a mechanosensory use for orientation. When the plumes were taped down, there was a 275% increase in the head bumping the sides of the maze.4
This Tufted Puffin excavates burrows up to 5 feet deep. Can the assumption be made that it too has mechanosensory plumes?

Tufted Puffin TUPU
Peafowl Crest
This section is a summary of a study on peafowl crests thus all paragraphs are cited to the same source.11
Studies in mammals and arthropods sensory hairs and antennae have found that they have mechanosensory roles in sound detection. Besides the enervation at the base, mechanical structures and vibrational response play a critical role. These structures are frequency-matched to the stimulus source like a caterpillar sensing an approaching wasp.
Bird feathers, like bristles and filoplumes, are known mechanosensors that enable birds to respond to mechanical stimuli. When sound related, feathers are frequency-tuned to their stimuli providing an advantage to filter irrelevant sounds. These feathers have a resonant frequency, vibrating at the maximum to the energy transferred by the sound wave they are tuned to.
Tests on peafowl crests demonstrate that they have mechanical properties. The resonant frequency of these feathers matches the frequencies emanating from tail rattling during social displays. The resulting hypothesis is that birds receive and respond to vibrotactile cues in a wider variety of scenarios.
Morphologically Similar
Research Details by Taxonomic Rank
Order definitions taken from Wikipidia
Struthioniformes
- an order of birds with only a single extant family, Struthionidae, containing the ostriches
Ostrich OSTR
Ostrich OSTR bristle auricular
Anseriformes
- an order of birds also known as waterfowl that comprises about 180 living species of birds in three families: Anhimidae, Anseranatidae, and Anatidae, the largest family, which includes over 170 species of waterfowl, among them the ducks, geese, and swans.
Northern Shoveler NSHO, dabbling duck
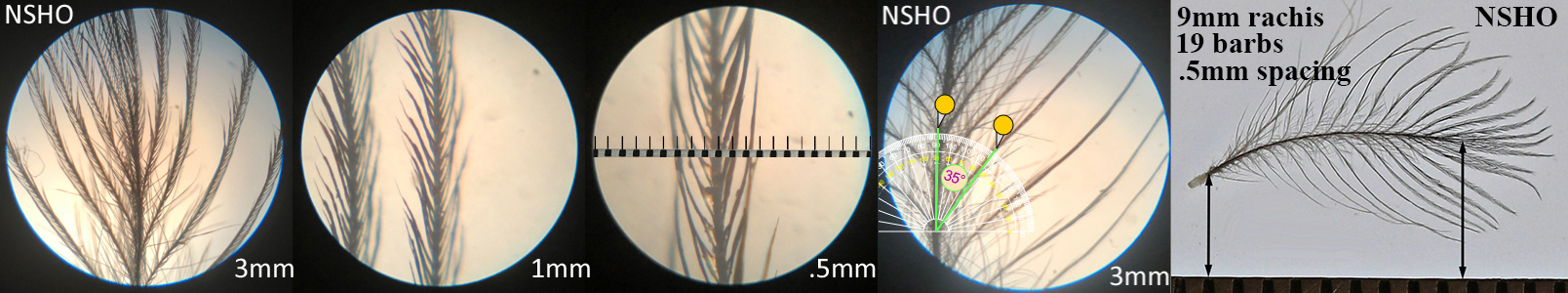
Northern Shoveler NSHO auricular details
American Wigeon AMWI, dabbling duck
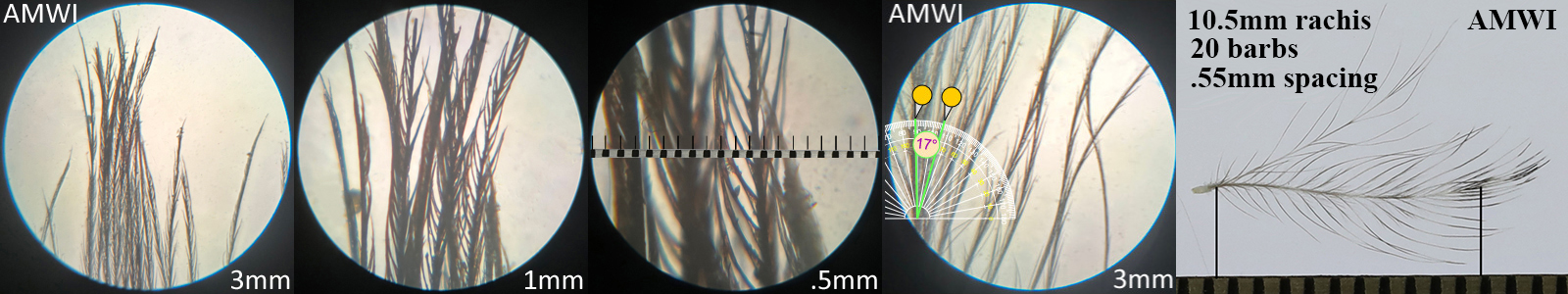
American Wigeon AMWI auricular details
Green-winged Teal GWTE, dabbling duck
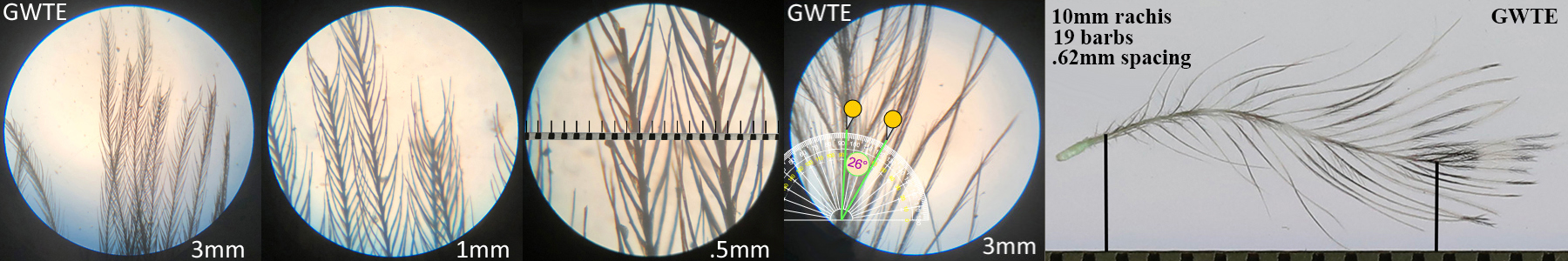
Green-winged Teal GWTE auricular details
Lesser Scaup LESC, diving duck
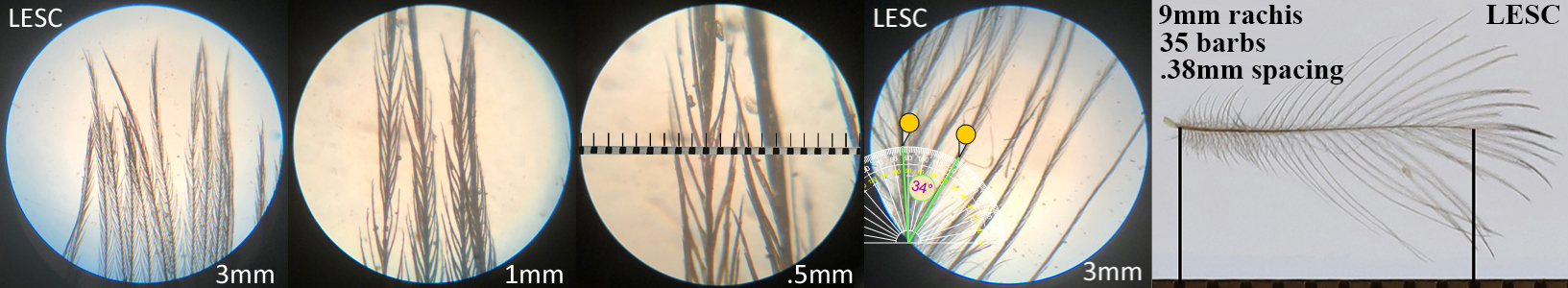
Lesser Scaup LESC auricular details
Common Goldeneye COGO, diving duck
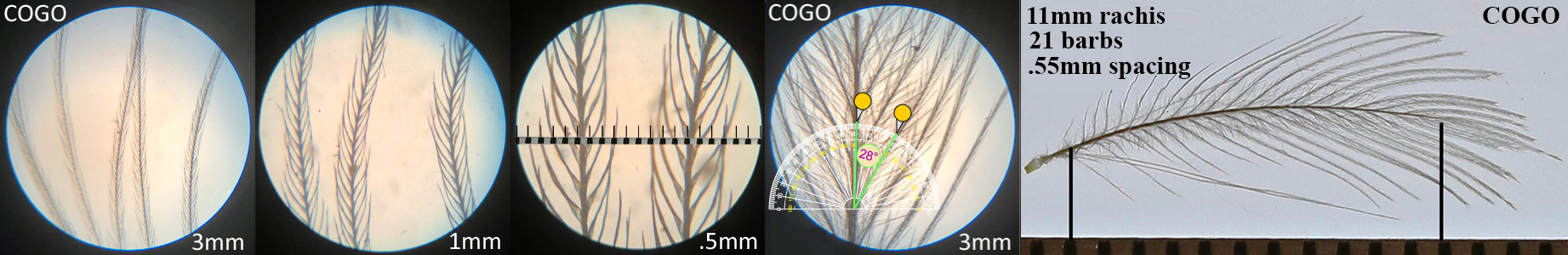
Common Goldeneye COGO auricular details
Galliformes
- an order of heavy-bodied ground-feeding birds that includes turkeys, chickens, quail, and other landfowl. Gallinaceous birds, as they are called, are important in their ecosystems as seed dispersers and predators, and are often reared by humans for their meat and eggs, or hunted as game birds.
Gambel’s Quail GAQU
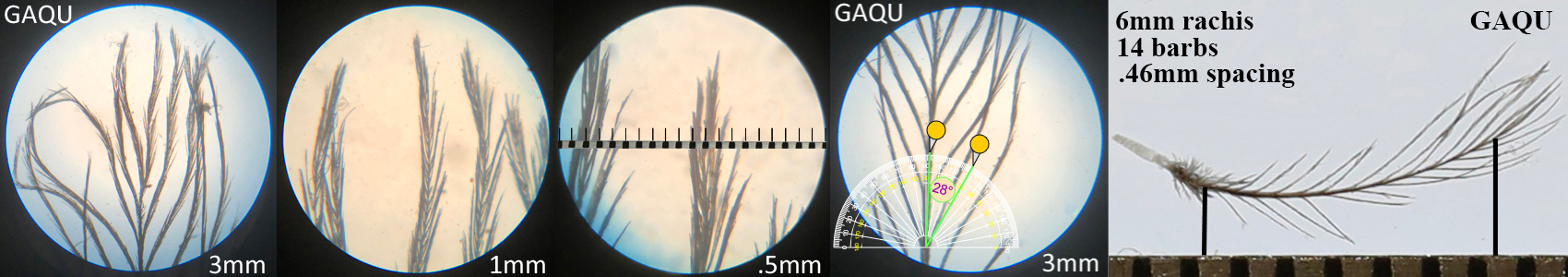
Gambel’s Quail GAQU auricular details
Wild Turkey WITU
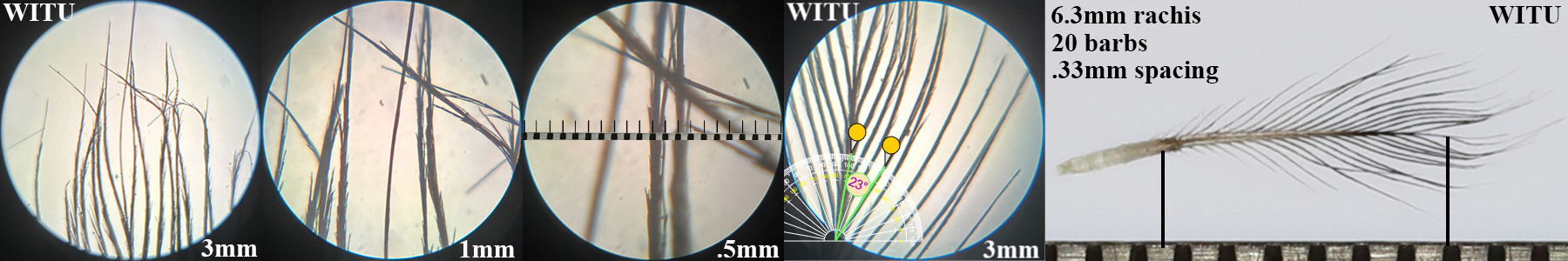
Wild Turkey WITU auricular details
Ring-necked Pheasant RNEP
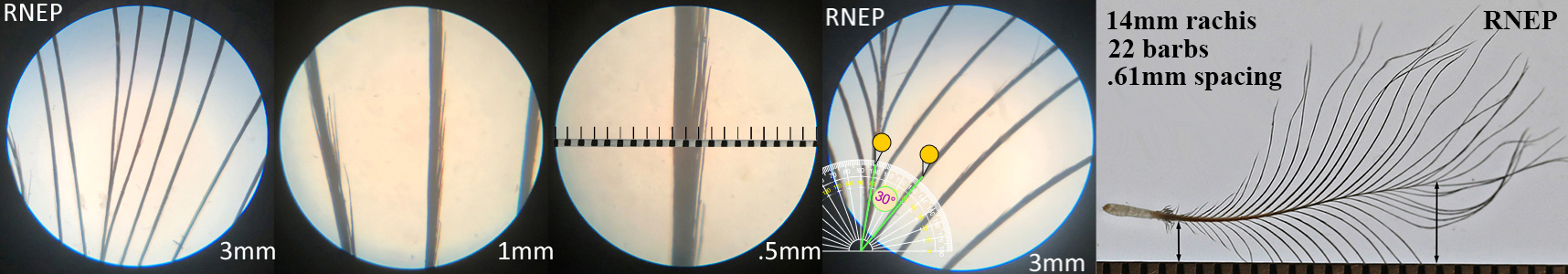
Ring-necked Pheasant RNEP auricular details
Chicken CHIC
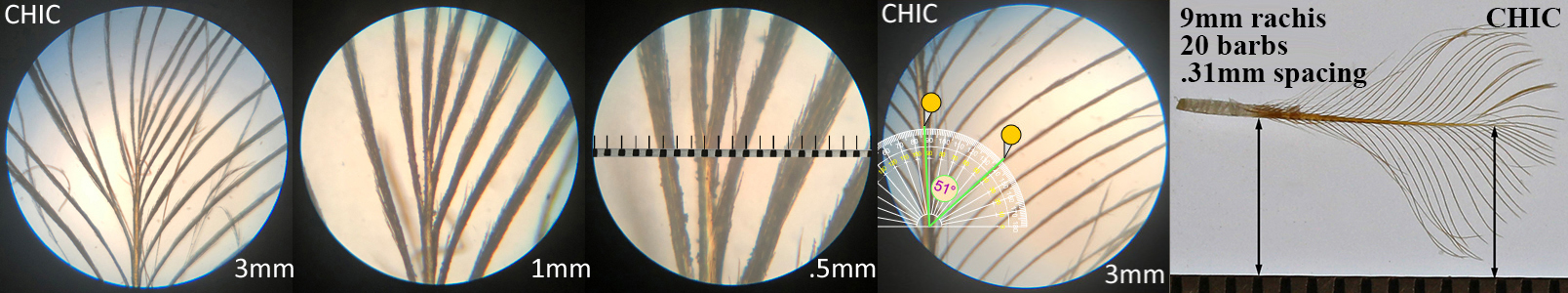
Chicken CHIC auricular details
Chukar CHUK
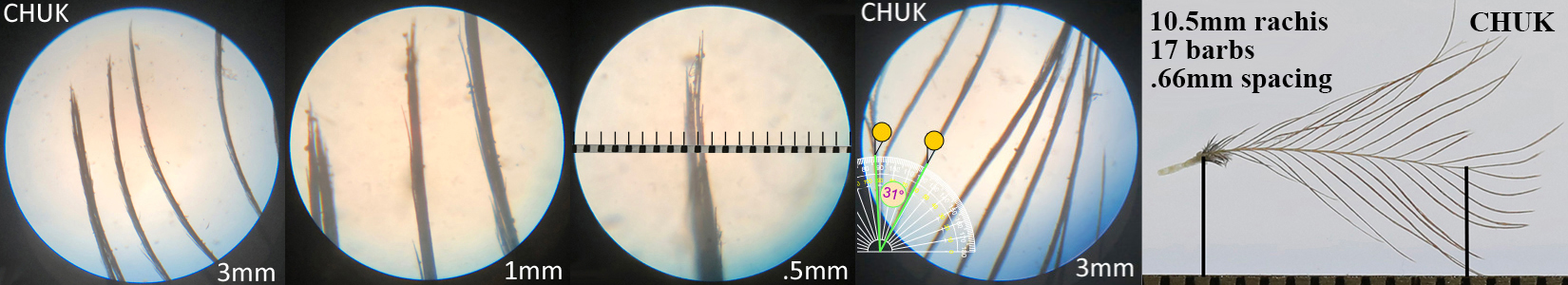
Chukar CHUK auricular details
Columbiformes
- an order of birds that includes the very widespread and successful doves and pigeons, classified in the family Columbidae
Rock Pigeon ROPI
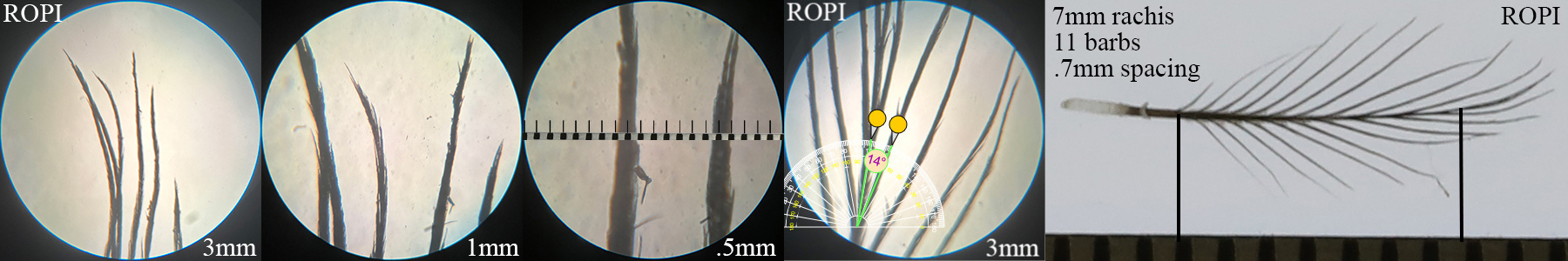
Rock Pigeon ROPI auricular details
White-winged Dove WWDO
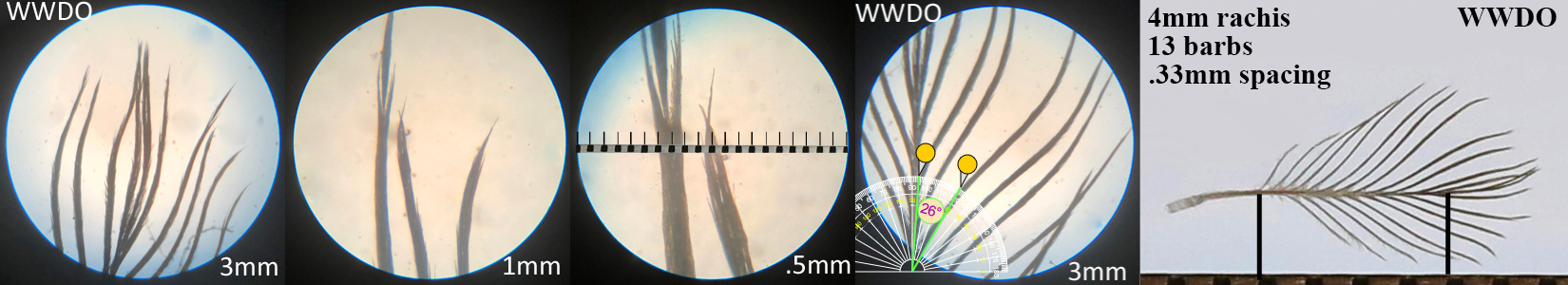
White-winged Dove WWDO auricular details
Mourning Dove MODO
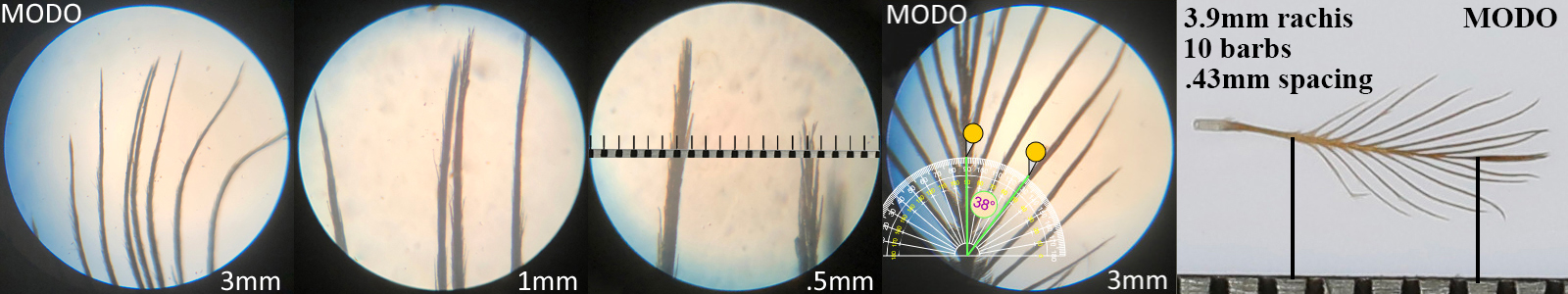
Mourning Dove MODO auricular details
Cuculiformes
Greater Roadrunner GRRO
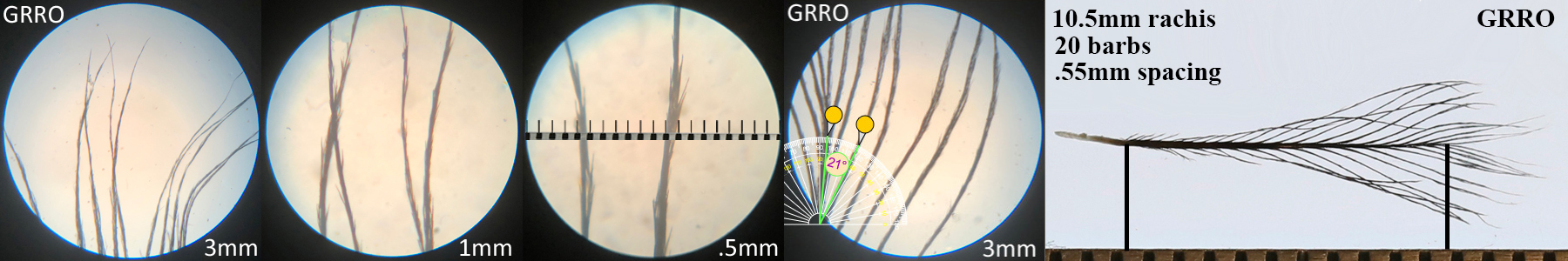
Greater Roadrunner GRRO auricular details
Apodiformes
- contains three living families: the swifts, the treeswifts, and the hummingbirds.
Black-chinned Hummingbird BCHU
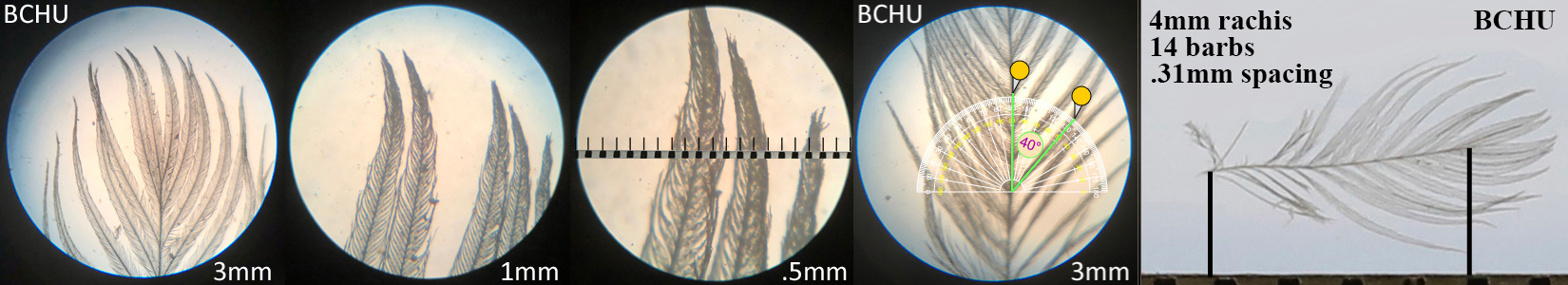
Black-chinned Hummingbird BCHU auricular details
Anna’s Hummingbird ANHU
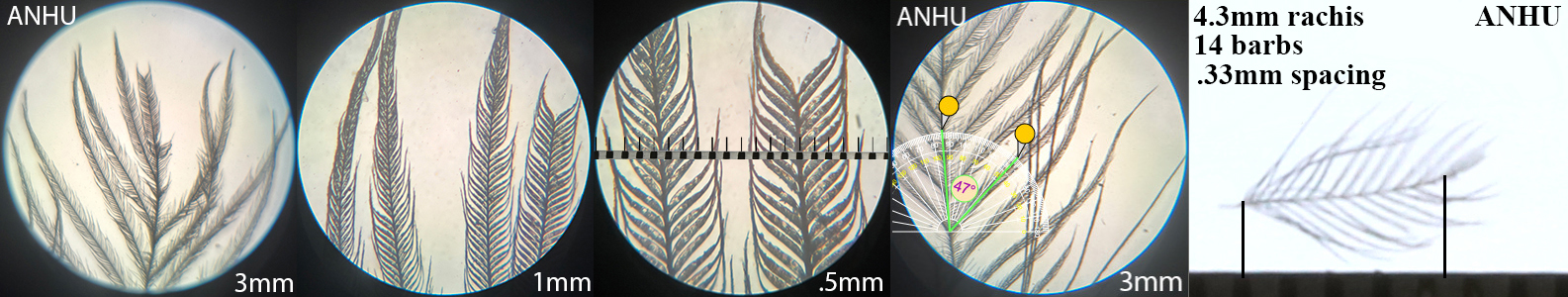
Anna’s Hummingbird ANHU auricular details
Costa’s Hummingbird COHU
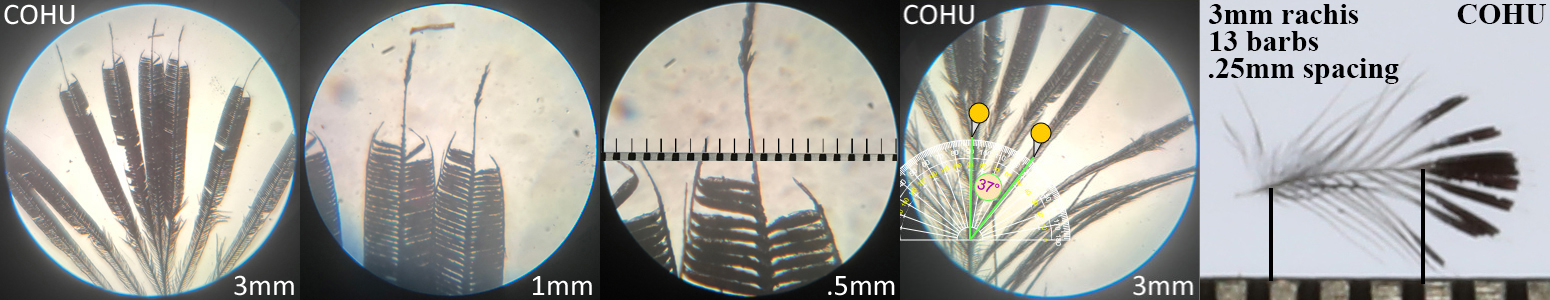
Costa’s Hummingbird COHU auricular details
Rufous Hummingbird RUHU AHYM

Rufous Hummingbird RUHU auricular details
Rufous Hummingbird RUHU HYF
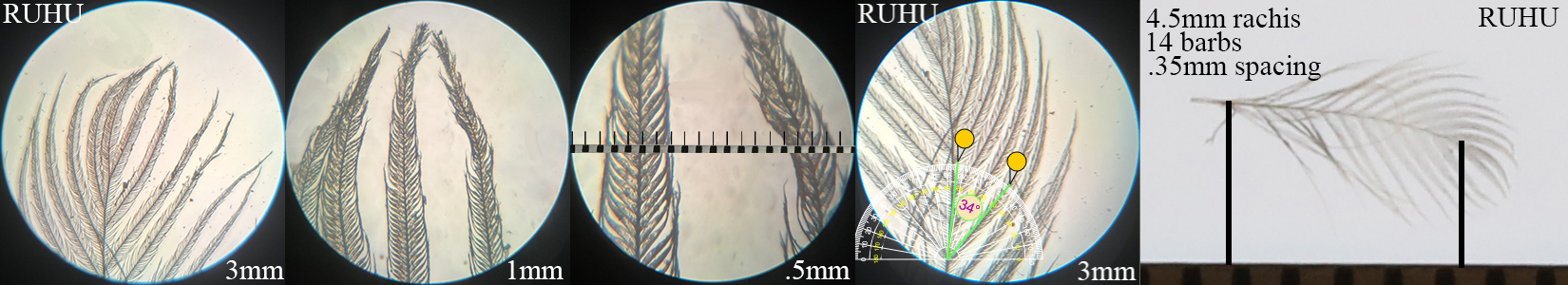
Rufous Hummingbird RUHU HYF auricular details
Gruiformes
- an order containing a considerable number of living and extinct bird families, with a widespread geographical diversity. Gruiform means “crane-like”. Traditionally, a number of wading and terrestrial bird families that did not seem to belong to any other order were classified together as Gruiformes.
Sandhill Crane SACR
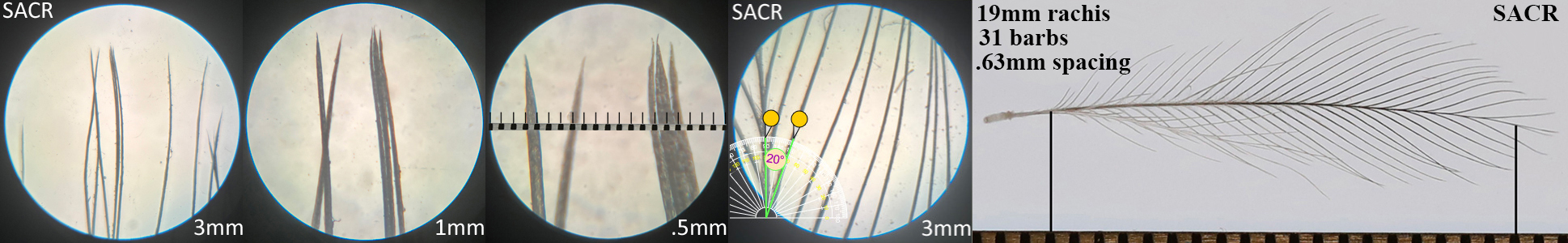
Sandhill Crane SACR auricular details
Charadriiformes
- a diverse order of small to medium-large birds. It includes about 390 species and has members in all parts of the world. Most charadriiform birds live near water and eat invertebrates or other small animals; however, some are pelagic, others frequent deserts, and a few are found in dense forest.
Wilson’s Snipe WISN
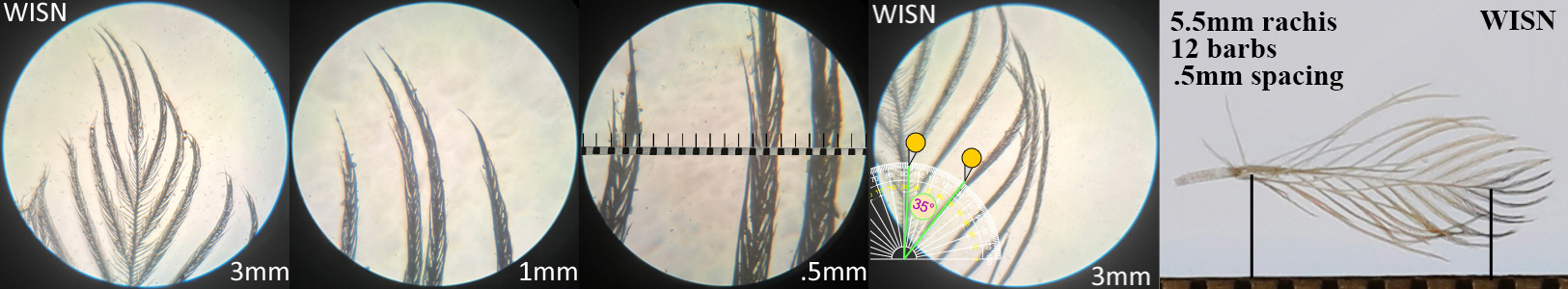
Wilson’s Snipe WISN auricular details
Accipitriformes
- an order of birds that includes most of the diurnal birds of prey, including hawks, eagles, vultures, and kites, but not falcons.
Sharp-shinned Hawk SSHA
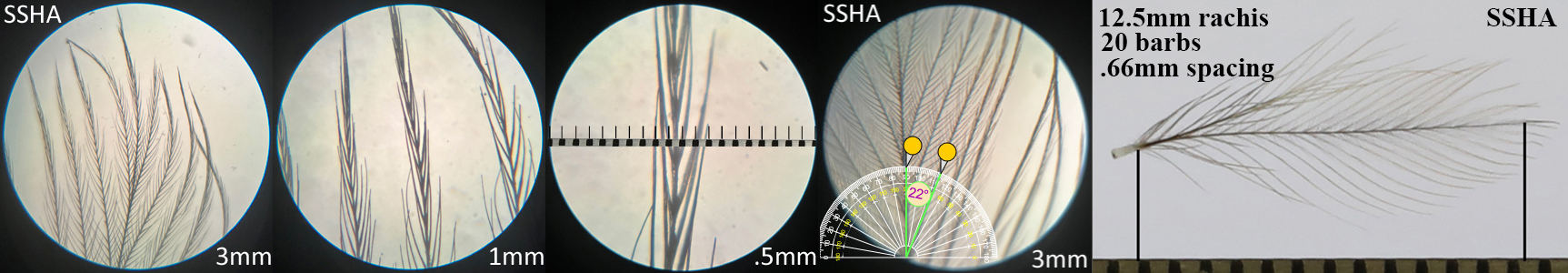
Sharpe-shinned Hawk SSHA auricular details
Cooper’s Hawk COHA
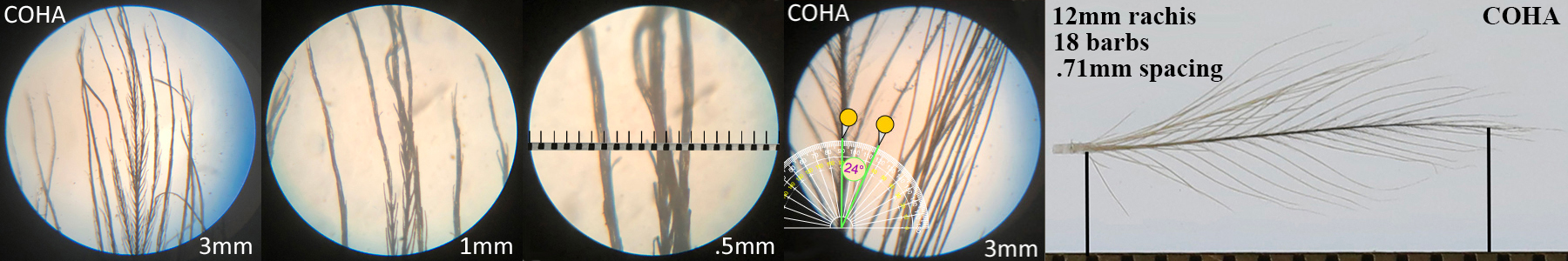
Cooper’s Hawk COHA auricular details
Harris’s Hawk HASH
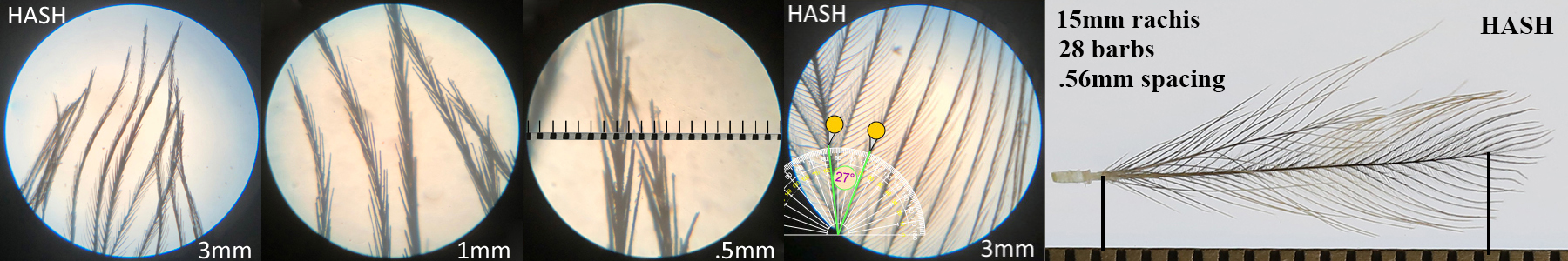
Harris’s Hawk HASH auricular details
Red-tailed Hawk RTHA
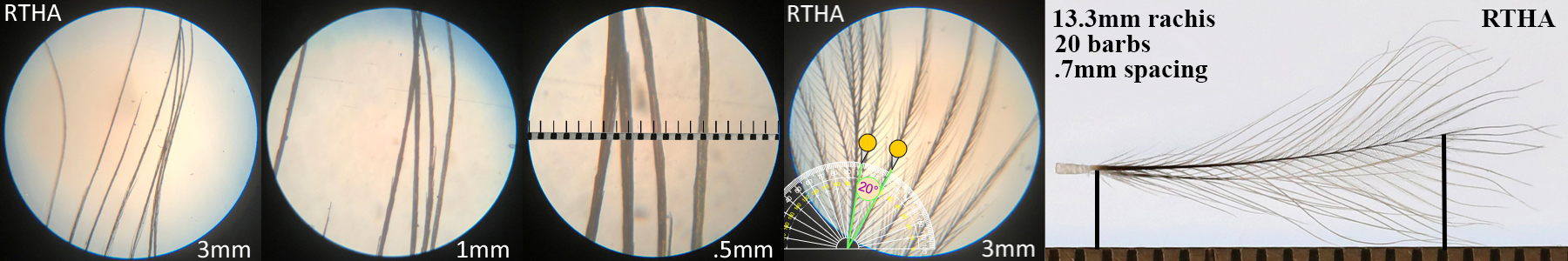
Red-tailed Hawk RTHA auricular details
Strigiformes
- Owls are birds from the order Strigiformes, which includes over 200 species of mostly solitary and nocturnal birds of prey typified by an upright stance, a large, broad head, binocular vision, binaural hearing, sharp talons, and feathers adapted for silent flight.
Barn Owl BANO
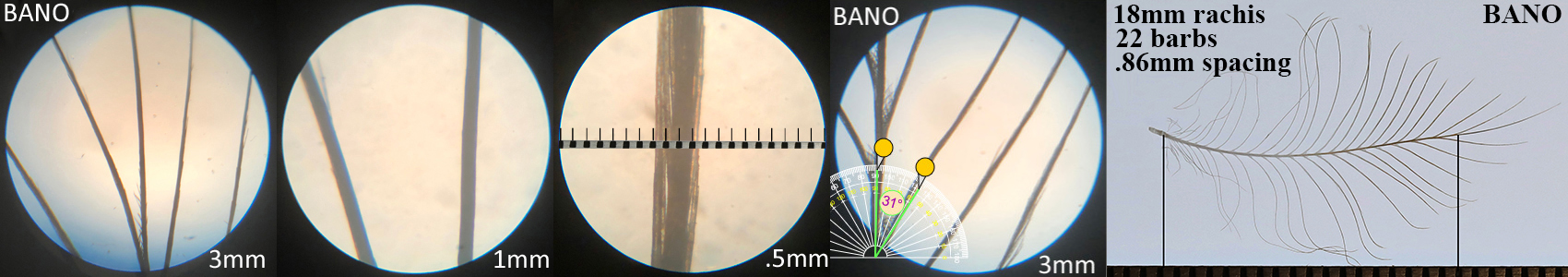
Barn Owl BANO auricular details
Flammulated Owl FLOW
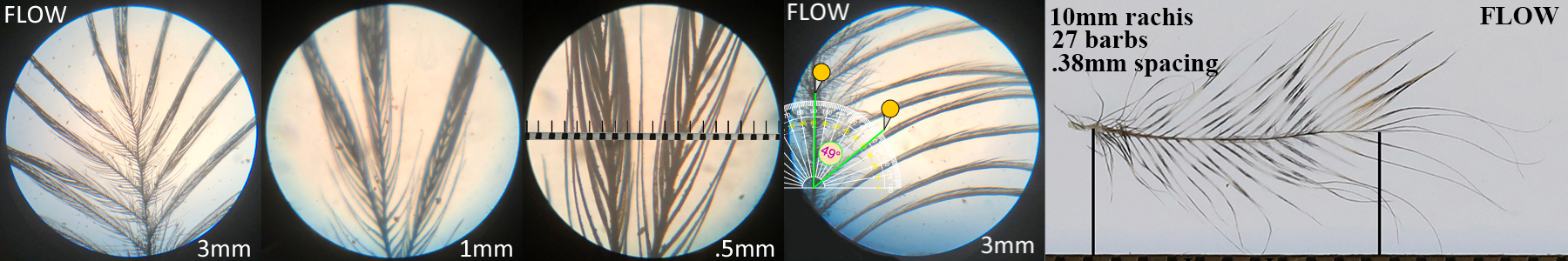
Flammulated Owl FLOW auricular details
Western Screech Owl WESO
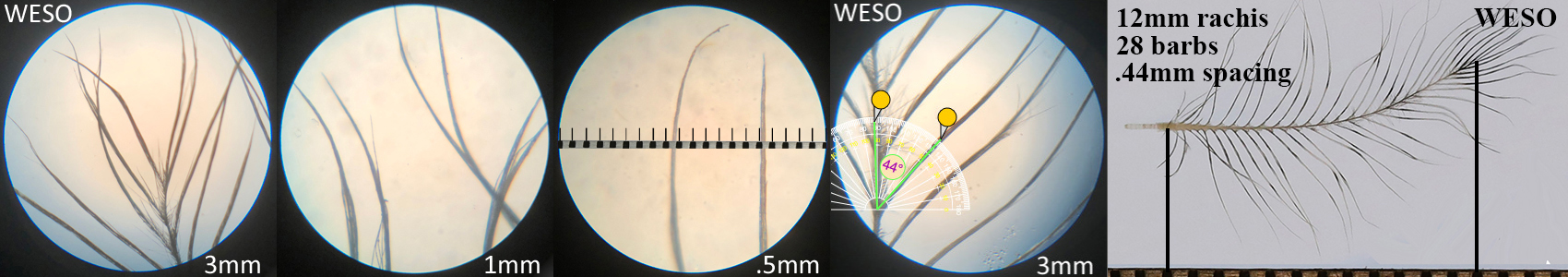
Western Screech Owl WESO auricular details
Great Horned Owl GHOW
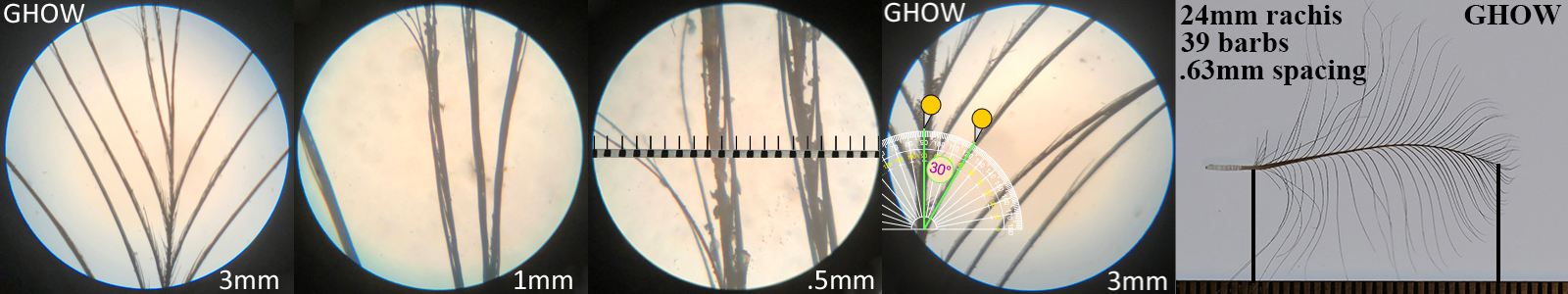
Great Horned Owl GHOW auricular details
Northern Pygmy Owl NOPO
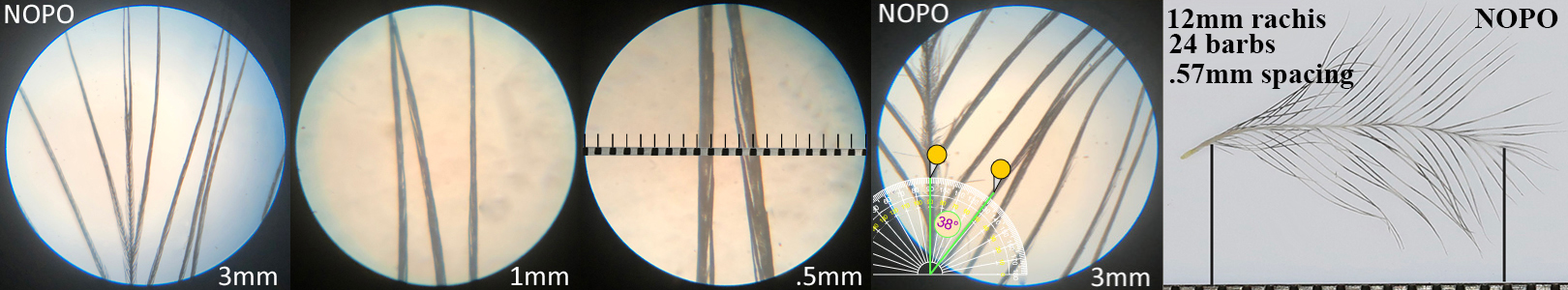
Northern Pygmy Owl NOPO auricular details
Ferruginous Pygmy Owl FEPO
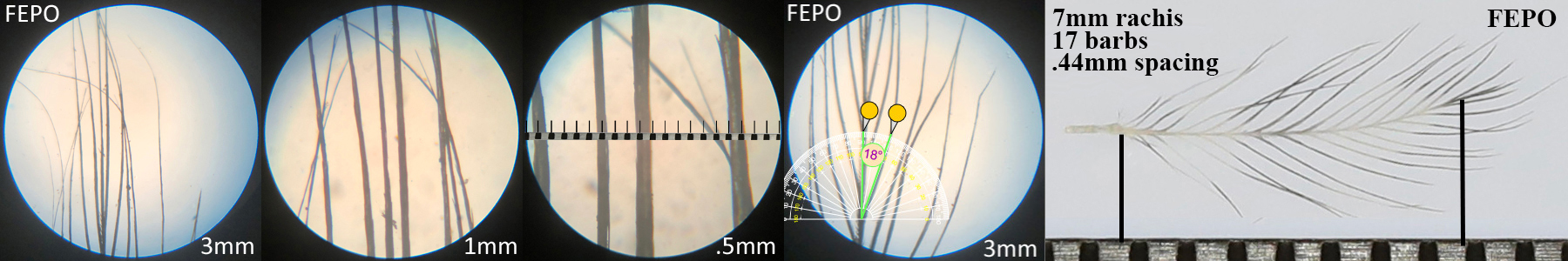
Ferruginous Pygmy Owl FEPO auricular details
Burrowing Owl BUOW
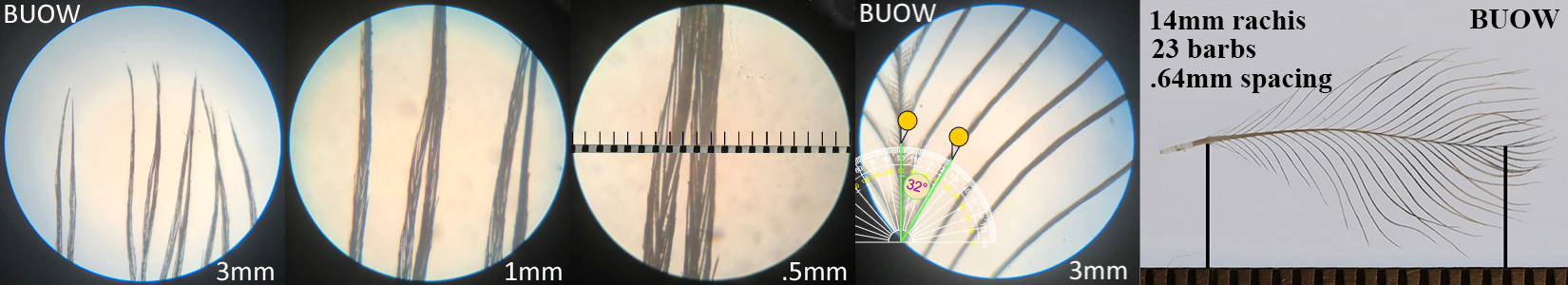
Burrowing Owl BUOW auricular details
Long-eared Owl LEOW
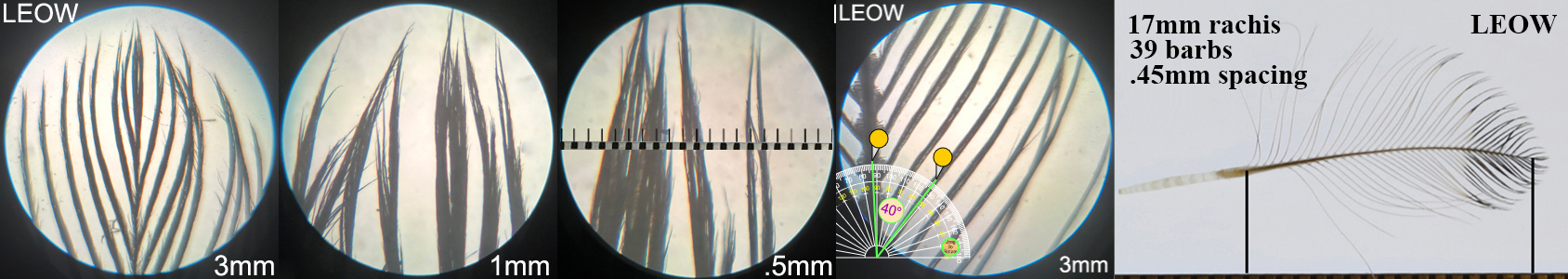
Long-eared Owl LEOW auricular details
Piciformes
- Nine families of largely arboreal birds make up the order Piciformes, the best-known of them being the Picidae, which includes the woodpeckers and close relatives. The Piciformes contain about 71 living genera with a little over 450 species, of which the Picidae make up about half.
Acorn Woodpecker ACWO
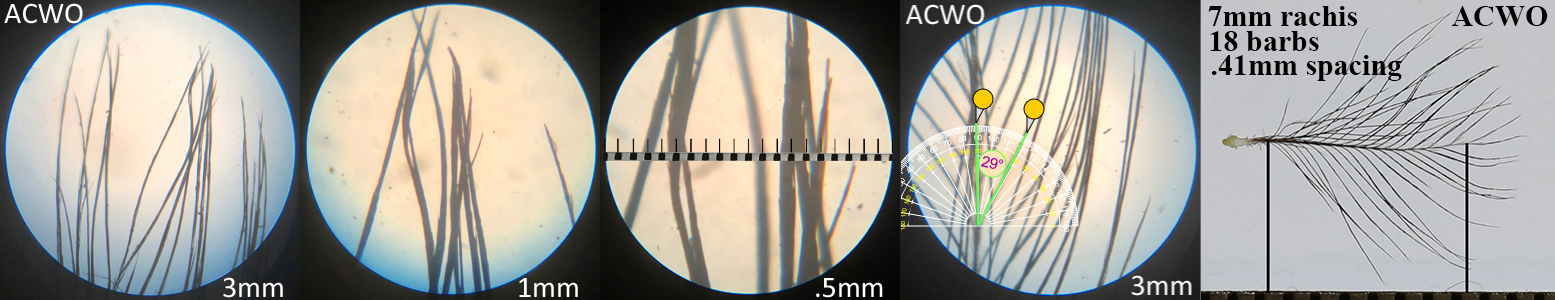
Acorn Woodpecker ACWO auricular details
Gila Woodpecker GIWO
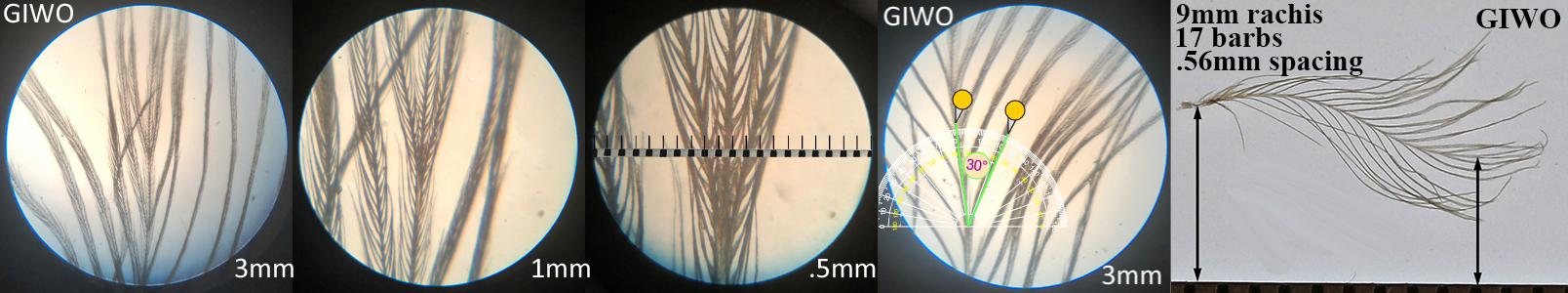
Gila Woodpecker GIWO auricular details
Falconiformes
- The order Falconiformes is represented by the extant family Falconidae and a handful of enigmatic Paleogene species. Traditionally, the other bird of prey families Cathartidae, Sagittariidae, Pandionidae, Accipitridae were classified in Falconiformes.
American Kestrel AMKE
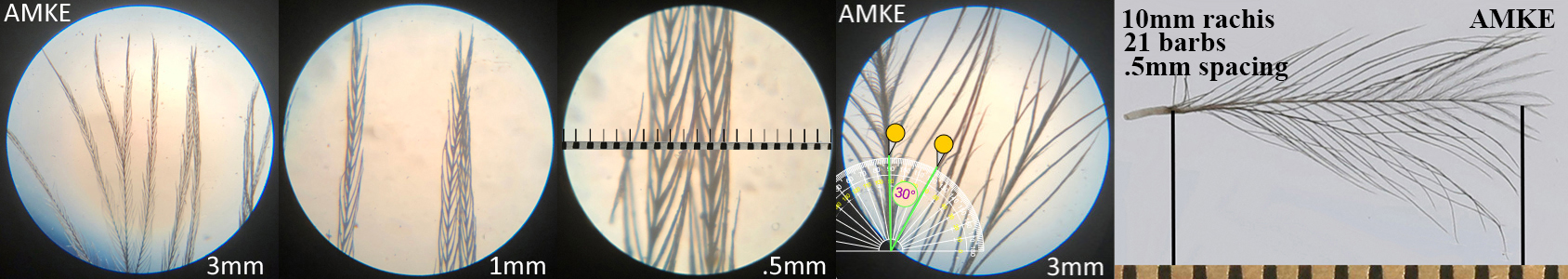
American Kestrel AMKE auricular details
Peregrine Falcon PEFA
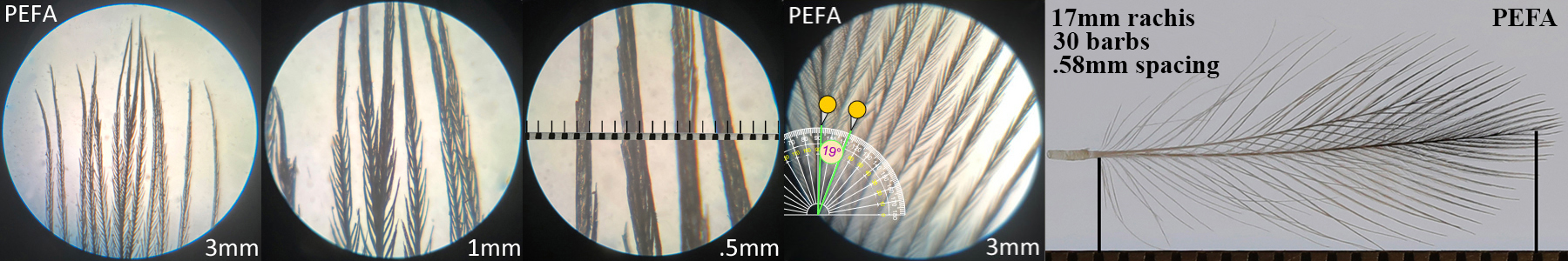
Peregrine Falcon PEFA auricular details
Psittaciformes
- Parrots, also known as psittacines, are birds with a strong curved beak, upright stance, and clawed feet. They are conformed by four families that contain roughly 410 species in 101 genera, found mostly in tropical and subtropical regions.
Rosy-faced Lovebird RFLO
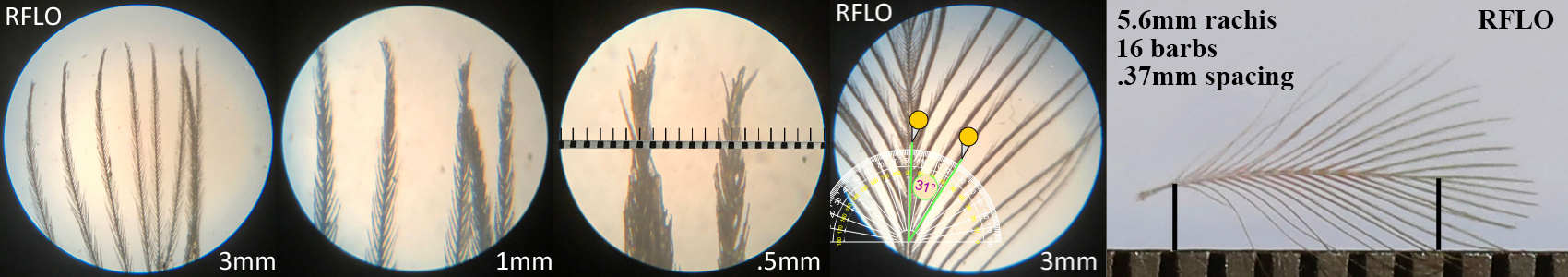
Rosy-faced Lovebird RFLO auricular details
Passeriformes
- A passerine is any bird of the order Passeriformes which includes more than half of all bird species. Sometimes known as perching birds, passerines generally have an anisodactyl (having the first toe directed backwards and the other three toes directed forwards) arrangement of their toes, which facilitates perching.
American Crow AMCR
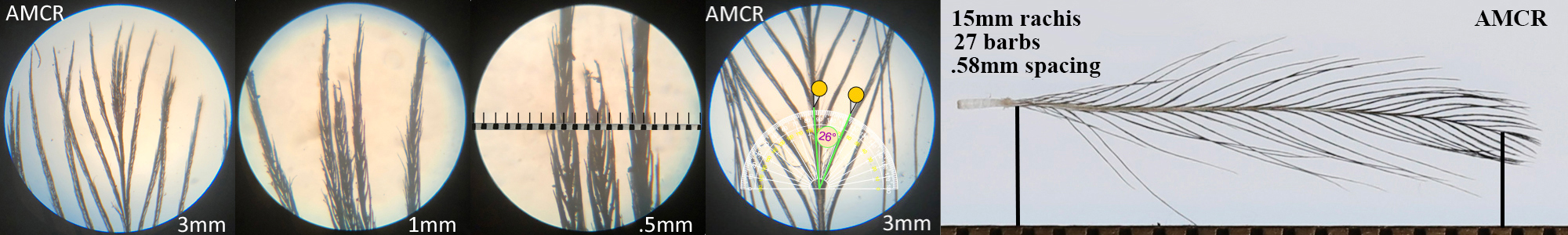
American Crow AMCR auricular details
W

White-breasted Nuthatch WBNU auricular details
Cactus Wren CACW
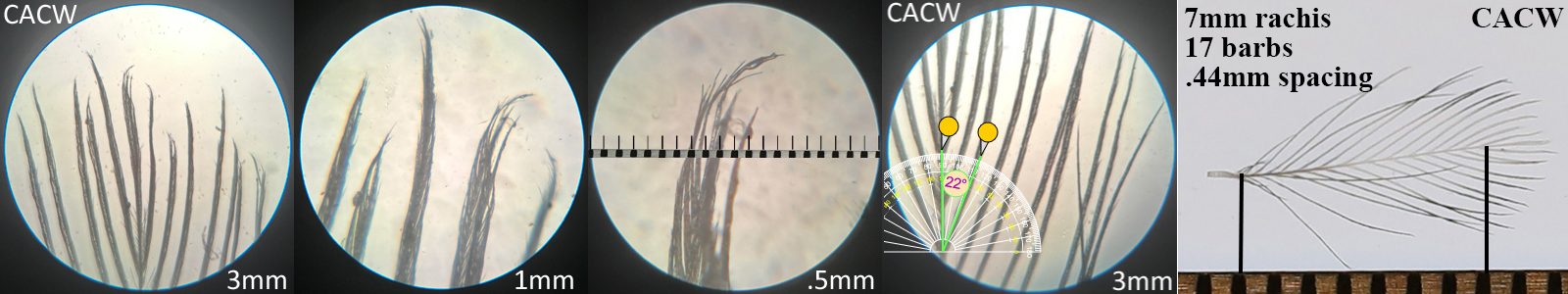
Cactus Wren CACW auricular details
Curve-billed Thrasher CBTH
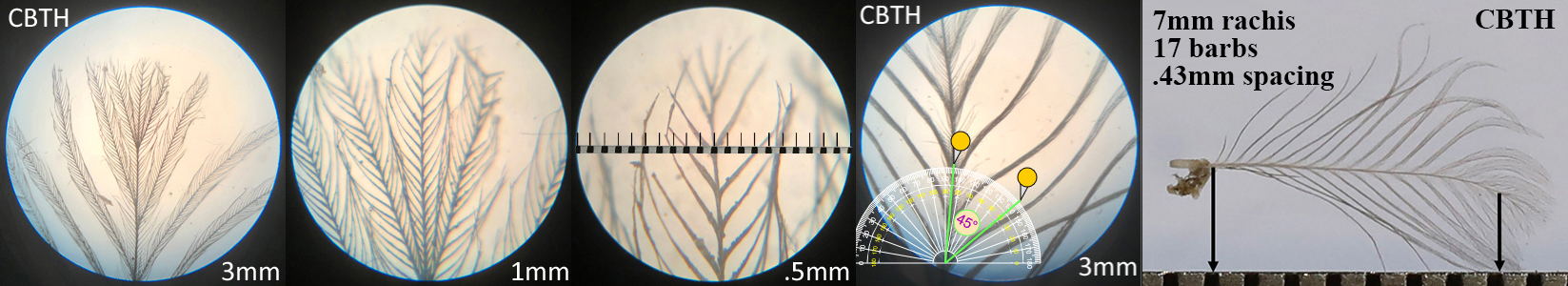
Curve-billed Thrasher CBTH auricular details
European Starling EUST Adult
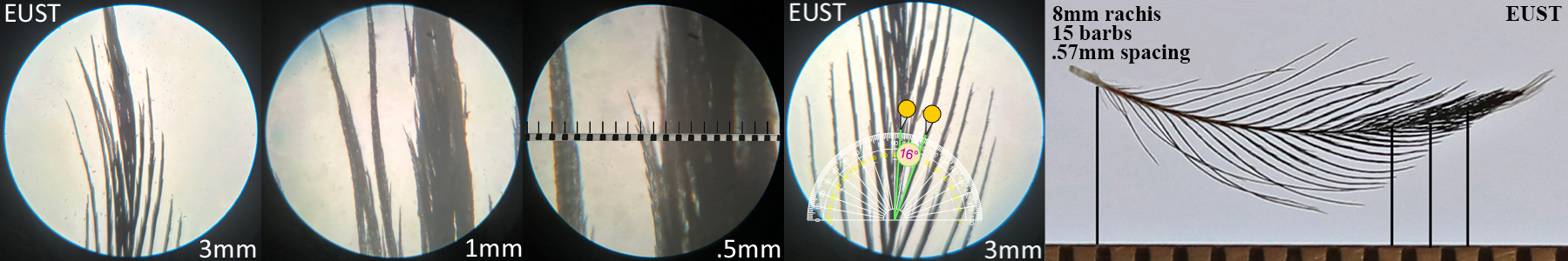
European Starling EUST adult profile
European Starling EUST Adult
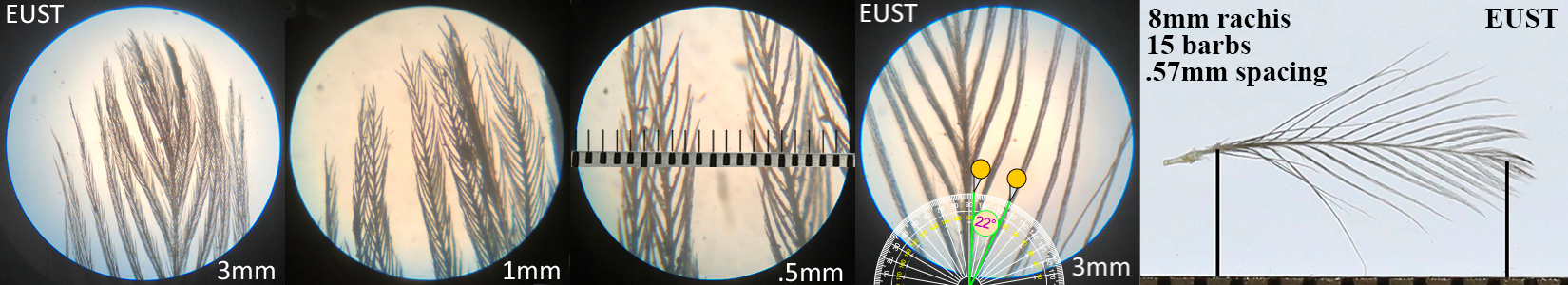
European Starling EUST juvenile auricular profile
Hermit Thrush HETH
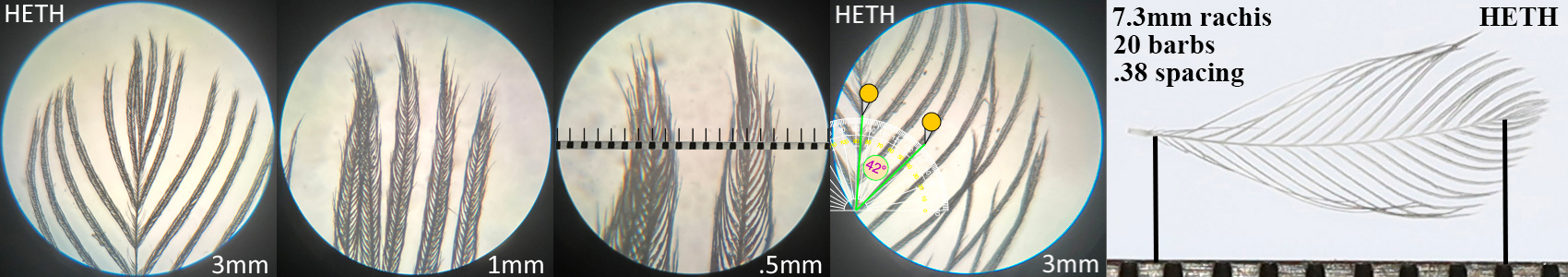
Hermit Thrush HETH auricular details
House Sparrow HOSP
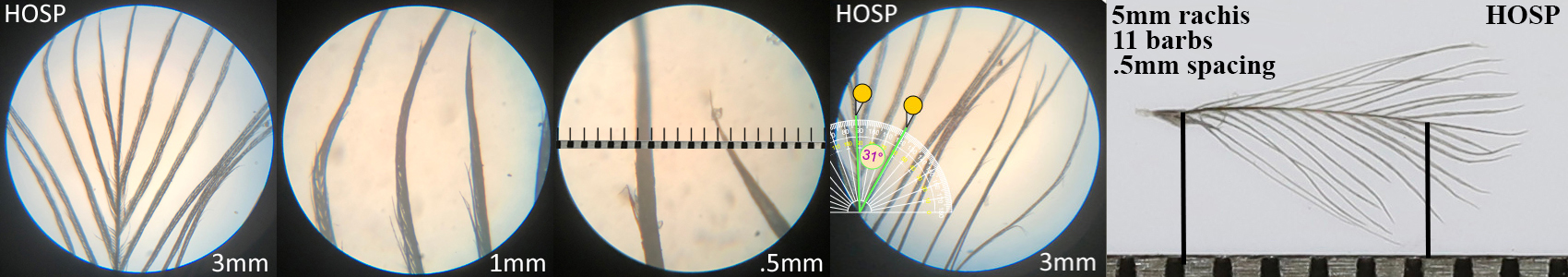
House Sparrow HOSP auricular details
House Finch HOFI
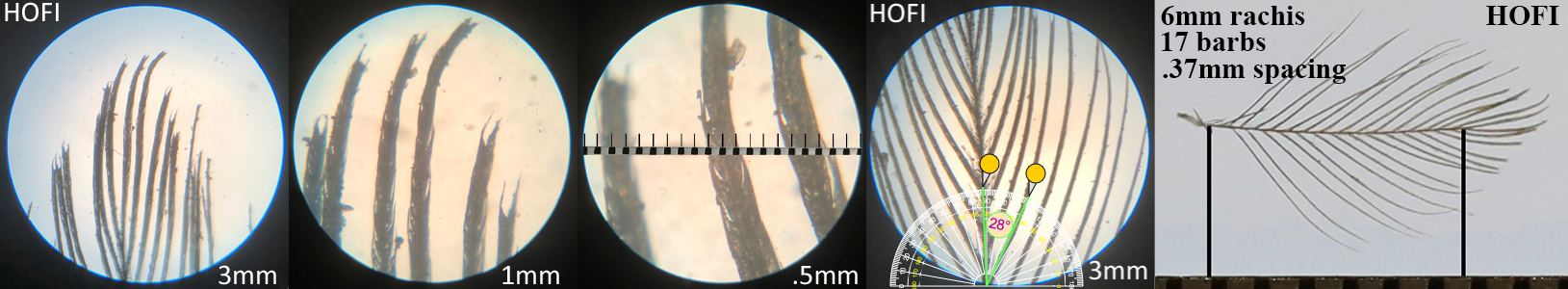
House Finch HOFI auricular details
Pine Siskin PISI
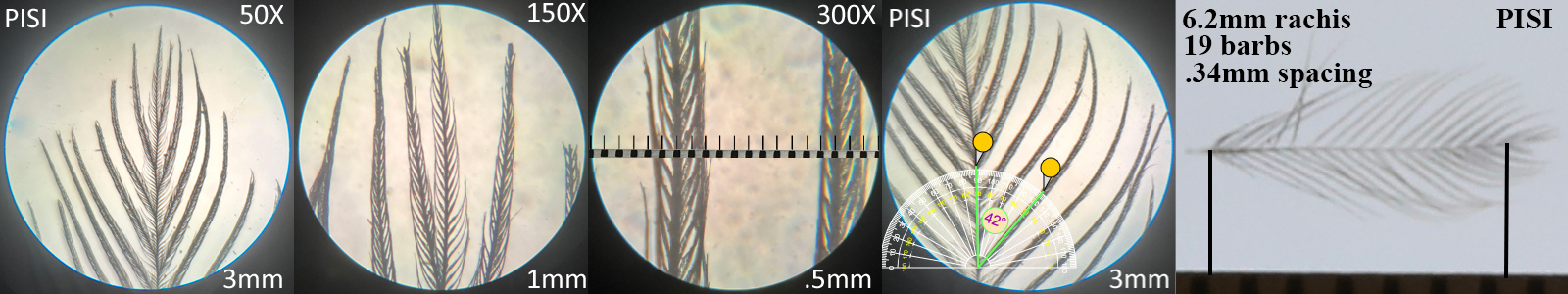
Pine Siskin PISI auricular details
Lesser Goldfinch LEGO
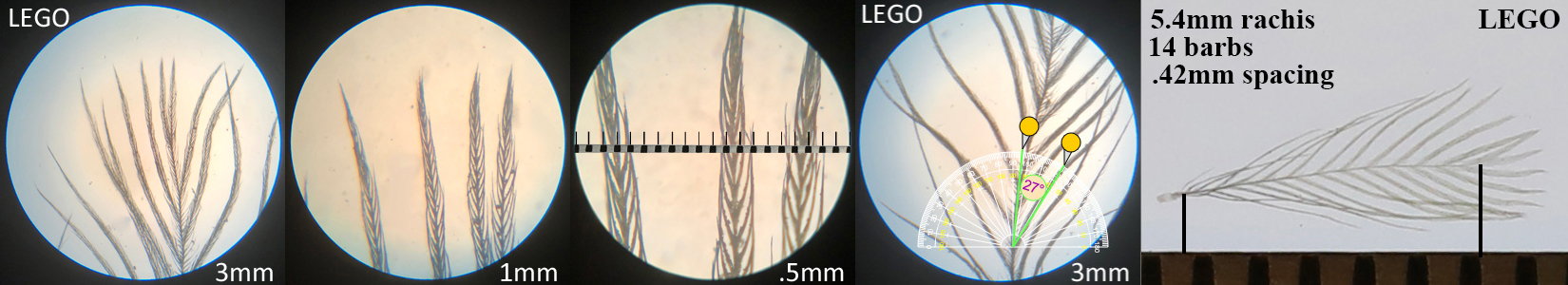
Lesser Goldfinch LEGO auricular details
Dark-eyed Junco DEJU
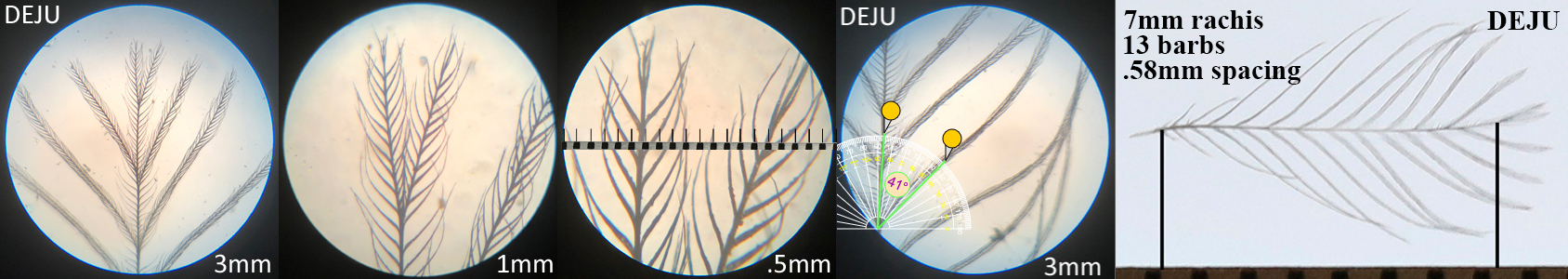
Dark-eyed Junco DEJU auricular details
Savannah Sparrow SAVS
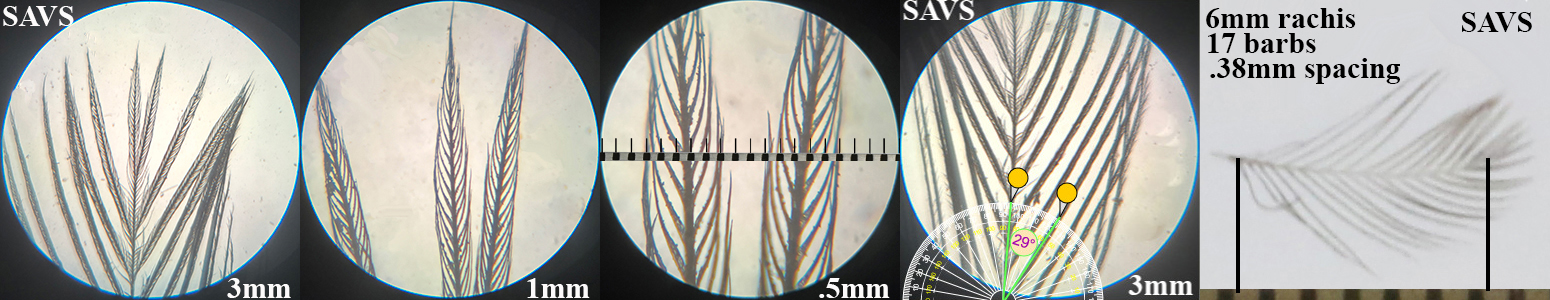
Savannah Sparrow SAVS auricular details
Abert’s Towhee ABTO
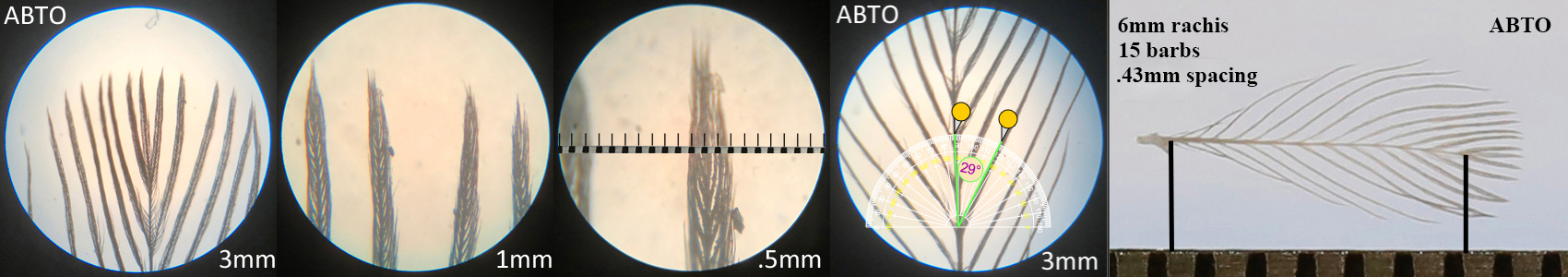
Abert’s Towhee ABTO auricular details
Yellow-rumped Warbler YRWA

Yellow-rumped Warbler YRWA auricular details
Northern Cardinal NOCA
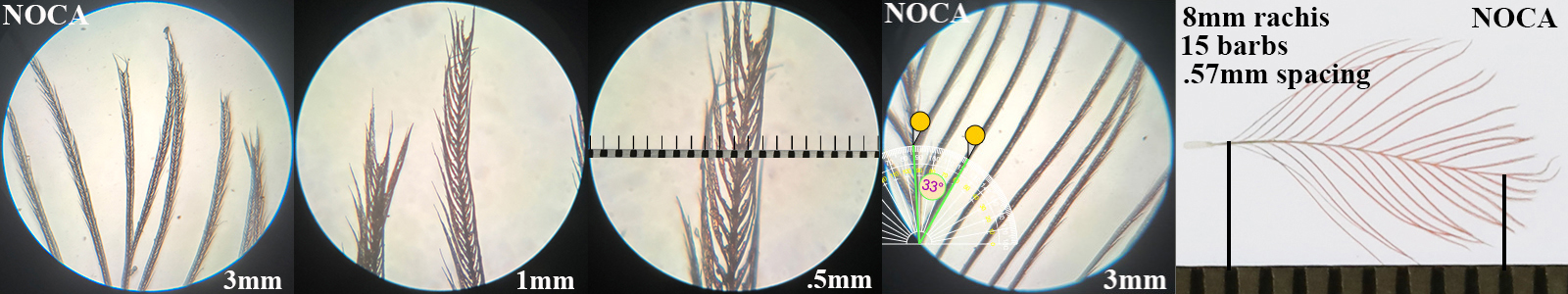
Northern Cardinal NOCA auricular details
Sonus Feathers Are Not Just Auriculares
In the Strigiformes order, sonus feathers are found throughout the facial disk. This Barn Owl is labeled where feather samples are taken.

Barn Owl BANO plucked auriculares placement
This Barn Owl feathergram displays the sampled feathers. Note none of the feathers were ear coverts.

Barn Owl BANO plucked auriculares placement
Positions 4 through 6 are bristle feathers. Positions 1, 2, 7, and 8 are all sonus variations. Eight owls are compared in the following feathergrams. The Ferruginous Pygmy Owl and Northern Pygmy Owl are both diurnal. Although the Burrowing Owl is considered crepuscular, its ruff feathers and its leading primary have diurnal traits.
Position 1 feather comparison
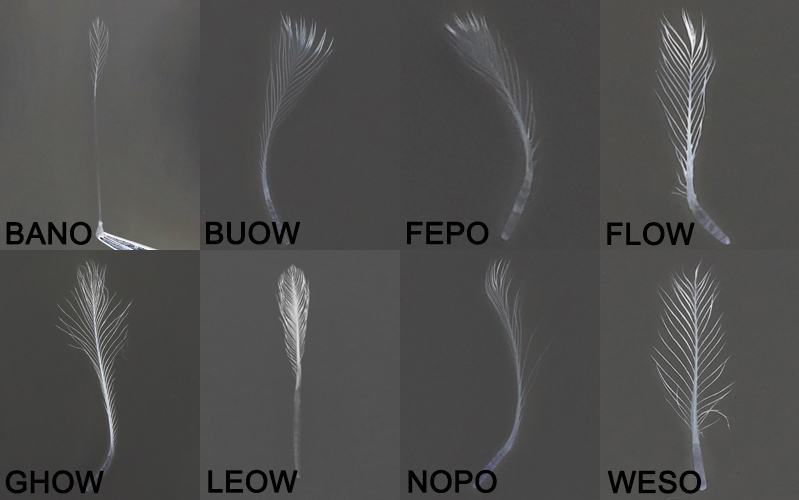
Position 1 Feathergram
Position 2 feather comparison
The night hunters clearly have sonus feathers on their cheeks.

Position 2 Feathergram
Position 7 feather comparison
The nocturnal species continue to show reliance on the sonus feathers above the eye.

Position 7 Feathergram
Position 8 feather comparison
In all four feathergrams the Barn Owl is very consistent with light and open feather structure. Its presence throughout the facial ruff indicates the importance of this feather structure in the Barn Owl’s sound sensitivity.

Position 8 Feathergram
Study Species
click on species to get study details
| Ind | Common Name | Code | Species | family | order |
| 1 | Ostrich | OSTR | Struthio camelus | Struthionidae | Struthioniformes |
| 29 | Northern Shoveler | NSHO | Spatula clypeata | Anatidae | Anseriformes |
| 33 | American Wigeon | AMWI | Mareca americana | Anatidae | Anseriformes |
| 43 | Green-winged Teal | GWTE | Anas crecca | Anatidae | Anseriformes |
| 50 | Lesser Scaup | LESC | Aythya affinis | Anatidae | Anseriformes |
| 64 | Common Goldeneye | COGO | Bucephala clangula | Anatidae | Anseriformes |
| 77 | Gambel’s Quail | GAQU | Callipepla gambelii | Odontophoridae | Galliformes |
| 79 | Wild Turkey | WITU | Meleagris gallopavo | Phasianidae | Galliformes |
| 93 | Ring-necked Pheasant | RNEP | Phasianus colchicus | Phasianidae | Galliformes |
| 94 | Chicken | CHIC | Gallus gallus | Phasianidae | Galliformes |
| 100 | Chukar | CHUK | Alectoris chukar | Phasianidae | Galliformes |
| 111 | Rock Pigeon | ROPI | Columba livia | Columbidae | Columbiformes |
| 129 | White-winged Dove | WWDO | Zenaida asiatica | Columbidae | Columbiformes |
| 131 | Mourning Dove | MODO | Zenaida macroura | Columbidae | Columbiformes |
| 134 | Greater Roadrunner | GRRO | Geococcyx californianus | Cuculidae | Cuculiformes |
| 169 | Black-chinned Hummingbird | BCHU | Archilochus alexandri | Trochilidae | Apodiformes |
| 171 | Anna’s Hummingbird | ANHU | Calypte anna | Trochilidae | Apodiformes |
| 172 | Costa’s Hummingbird | COHU | Calypte costae | Trochilidae | Apodiformes |
| 174 | Rufous Hummingbird | RUHU | Selasphorus rufus | Trochilidae | Apodiformes |
| 207 | Sandhill Crane | SACR | Antigone canadensis | Gruidae | Gruiformes |
| 283 | Wilson’s Snipe | WISN | Gallinago delicata | Scolopacidae | Charadriiformes |
| 521 | Sharp-shinned Hawk | SSHA | Accipiter striatus | Accipitridae | Accipitriformes |
| 522 | Cooper’s Hawk | COHA | Accipiter cooperii | Accipitridae | Accipitriformes |
| 534 | Harris’s Hawk | HASH | Parabuteo unicinctus | Accipitridae | Accipitriformes |
| 543 | Red-tailed Hawk | RTHA | Buteo jamaicensis | Accipitridae | Accipitriformes |
| 547 | Barn Owl | BANO | Tyto alba | Tytonidae | Strigiformes |
| 549 | Flammulated Owl | FLOW | Psiloscops flammeolus | Strigidae | Strigiformes |
| 551 | Western Screech Owl | WESO | Megascops kennicottii | Strigidae | Strigiformes |
| 553 | Great Horned Owl | GHOW | Bubo virginianus | Strigidae | Strigiformes |
| 556 | Northern Pygmy Owl | NOPO | Glaucidium gnoma | Strigidae | Strigiformes |
| 557 | Ferruginous Pygmy Owl | FEPO | Glaucidium brasilianum | Strigidae | Strigiformes |
| 559 | Burrowing Owl | BUOW | Athene cunicularia | Strigidae | Strigiformes |
| 564 | Long-eared Owl | LEOW | Asio otus | Strigidae | Strigiformes |
| 580 | Acorn Woodpecker | ACWO | Melanerpes formicivorus | Picidae | Piciformes |
| 581 | Gila Woodpecker | GIWO | Melanerpes uropygialis | Picidae | Piciformes |
| 605 | American Kestrel | AMKE | Falco sparverius | Falconidae | Falconiformes |
| 612 | Peregrine Falcon | PEFA | Falco peregrinus | Falconidae | Falconiformes |
| 626 | Rosy-faced Lovebird | RFLO | Agapornis roseicollis | Psittaculidae | Psittaciformes |
| 716 | American Crow | AMCR | Corvus brachyrhynchos | Corvidae | Passeriformes |
| 792 | White-breasted Nuthatch | WBNU | Sitta carolinensis | Sittidae | Passeriformes |
| 803 | Cactus Wren | CACW | Campylorhynchus brunneicapillus | Troglodytidae | Passeriformes |
| 813 | Curve-billed Thrasher | CBTH | Toxostoma curvirostre | Mimidae | Passeriformes |
| 823 | European Starling | EUST | Sturnus vulgaris | Sturnidae | Passeriformes |
| 842 | Hermit Thrush | HETH | Catharus guttatus | Turdidae | Passeriformes |
| 887 | House Sparrow | HOSP | Passer domesticus | Passeridae | Passeriformes |
| 950 | House Finch | HOFI | Haemorhous mexicanus | Fringillidae | Passeriformes |
| 961 | Pine Siskin | PISI | Spinus pinus | Fringillidae | Passeriformes |
| 962 | Lesser Goldfinch | LEGO | Spinus psaltria | Fringillidae | Passeriformes |
| 999 | Dark-eyed Junco | DEJU | Junco hyemalis | Passerellidae | Passeriformes |
| 1014 | Savannah Sparrow | SAVS | Passerculus sandwichensis | Passerellidae | Passeriformes |
| 1019 | Abert’s Towhee | ABTO | Melozone aberti | Passerellidae | Passeriformes |
| 1093 | Yellow-rumped Warbler | YRWA | Setophaga coronata | Parulidae | Passeriformes |
| 1116 | Northern Cardinal | NOCA | Cardinalis cardinalis | Cardinalidae | Passeriformes |
General Methods
Prior to starting the study I obtained a Scientific Activity License from the state of Arizona that is good for one year and can be renewed. The fee is $70/year. In order to get access to birds from a rehab facility I was requested to get a federal license. The federal license is good for three years at a cost of $100. Both licenses require annual reporting. The possession of feathers and other parts of native North American birds without a permit is prohibited by the Migratory Bird Treaty Act (MBTA). There is no exemption for molted feathers, birds that hit windows or road kill.

Scientific Activity License
Record provenance of each carcass collected which will be used in the annual report.
A note about measurements. All of the measurements are approximate and used to make broad observations. Other than the use of a caliper, ruler measurements round to the nearest millimeter.
Biometrics
Length of the bird

Morning Dove MODO length
Length of the primary

Cactus Wren CACW P10
Skull volume — length of skull is measured from the base of the maxilla (upper bill) to the back of the skull. Width and height measured at the maximum of each.
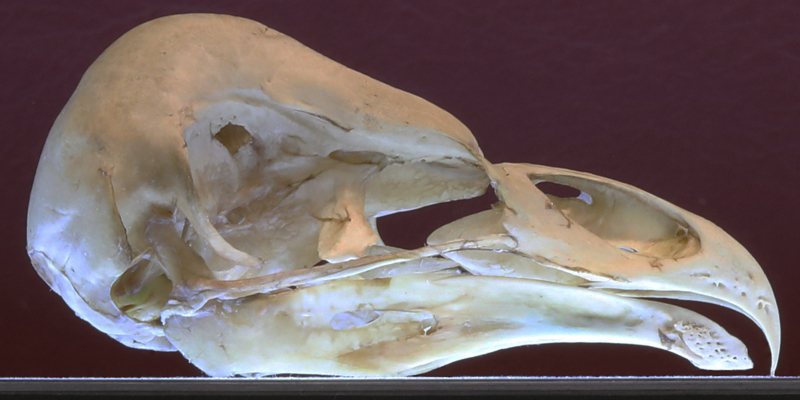
Barn Owl BANO skull
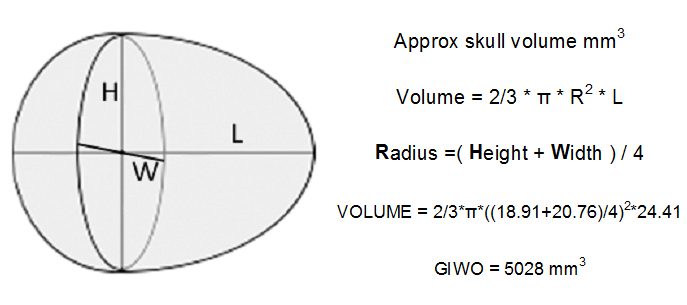
skull volume calculation
Meatus area

Ring-necked Pheasant RNEP meatus
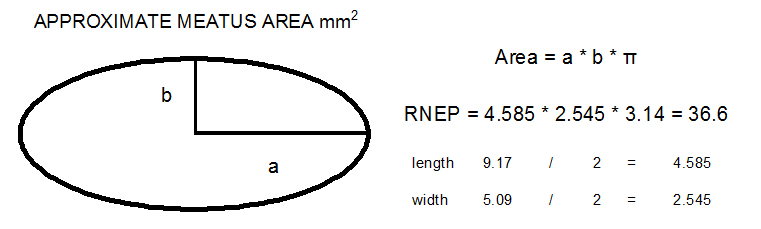
meatus area calculation
Eye orbit to meatus — the distance from the edge of the eye orbit to the meatus is measured. When working with a northern shoveler I notice that the meatus was significantly further away from the eye than the 3 other, non aquatic, species showed.
An ancillary task is accounting for any bristles. Bristles are an important subset of data for this study. Their relevance will be explained in the analysis section.
click here for photos of bristle feathers

Red-tailed Hawk RTHA bristle feathers

Red-tailed Hawk RTHA bristle feathers
Close up picture of the auricular area is taken.

RNEP GIWO auriculares
If an owl, a picture is taken of the facial disk if a close-up field photo isn’t available. The photo is used to map feather sampling.

Northern Pygmy-Owl NOPO facial feather location
Pluck auriculares
Auricular feathers were plucked from the closest to meatus out. The plucking stopped when the tip of the next feather no longer covers any part of the meatus.
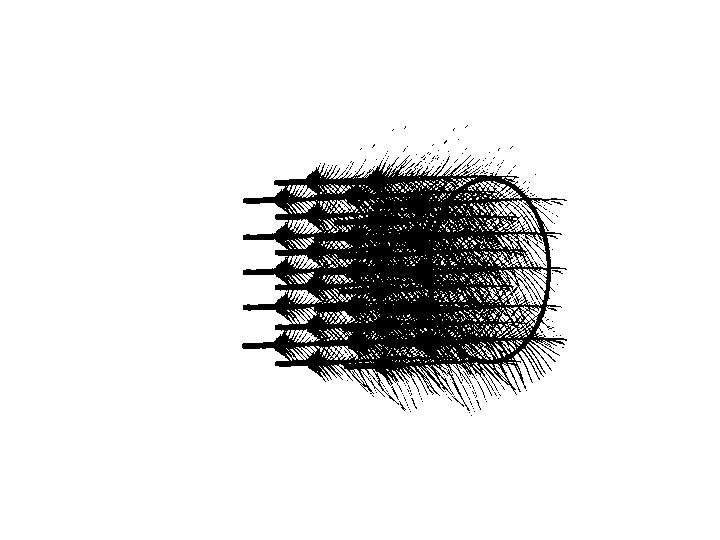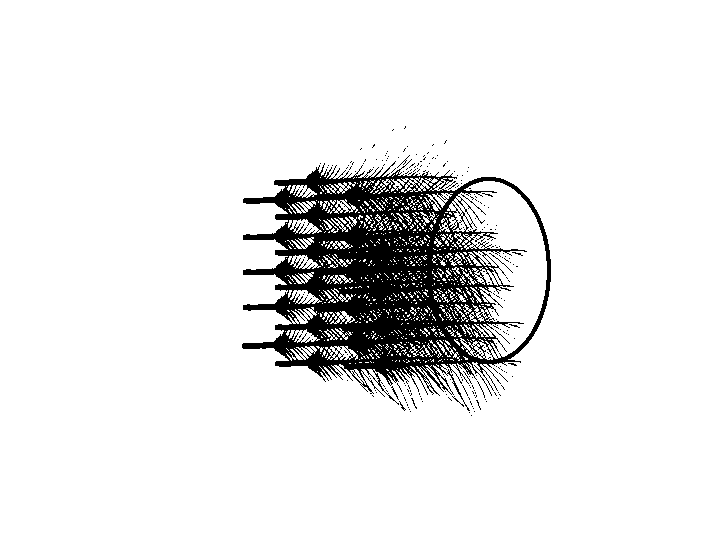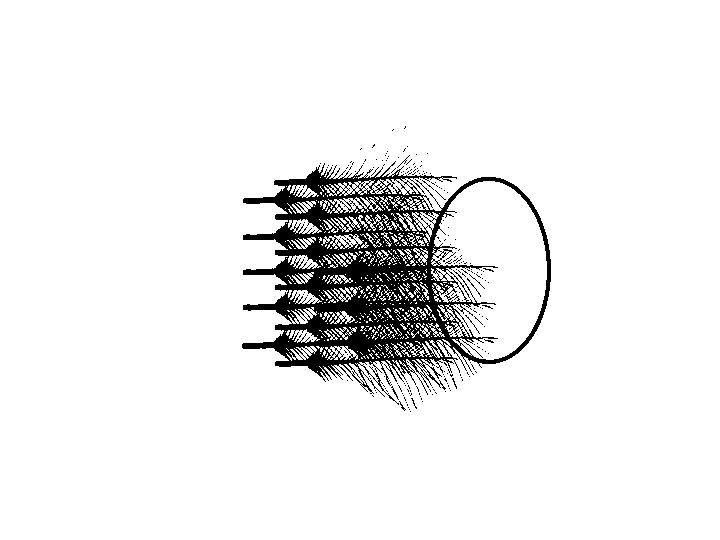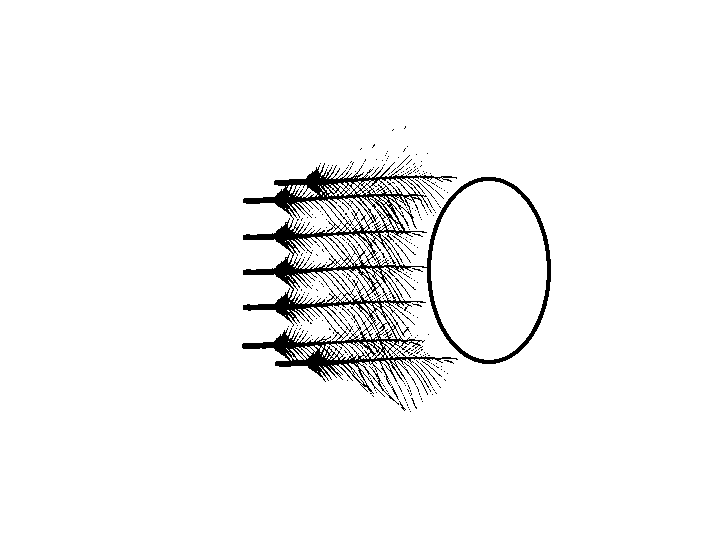
removing auricular feathers
Feathers are placed on a photo album page in the approximate order they were plucked. Plucked feathers are counted. Some birds have relatively uniform feather length while others have a mixture of short and long feathers. The feather count is subjective, counting large and small feathers (with period in front of the number).

Acorn Woodpecker ACWO plucked auriculares
Feather measurements
Sonus feather length and width
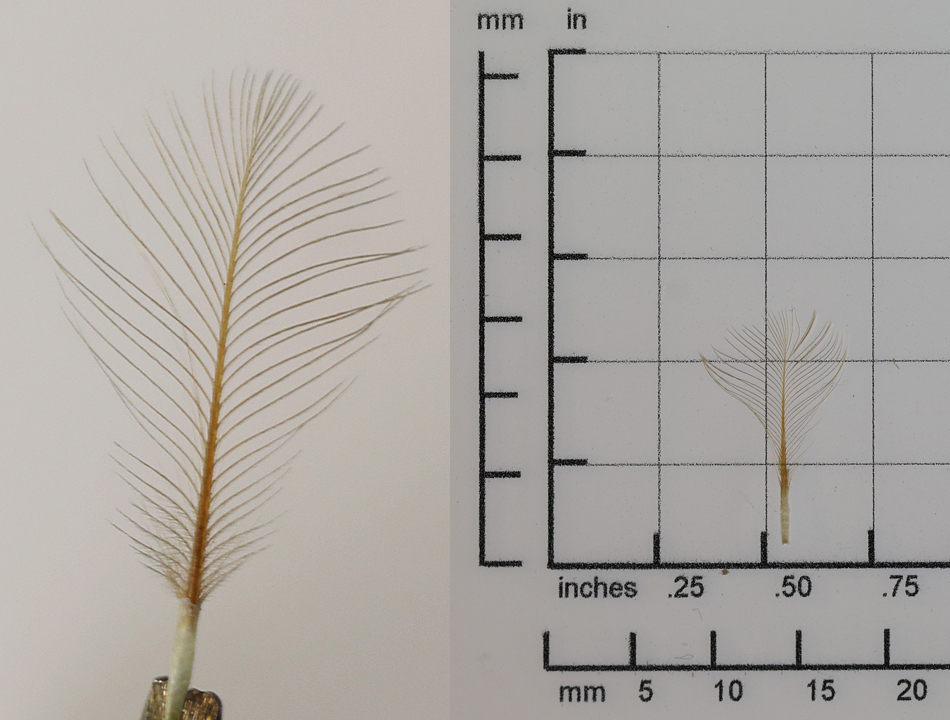
chicken sonus auricular
Barb spacing = rachis length / (number of barbs on one side – 1)
The rachis is measured. The barb pairs are counted in two groups, the shorter ones (S-count) starting at the calamus up to the first full length barb. The longer ones (L-count) are the remainder up to the tip of the feather.
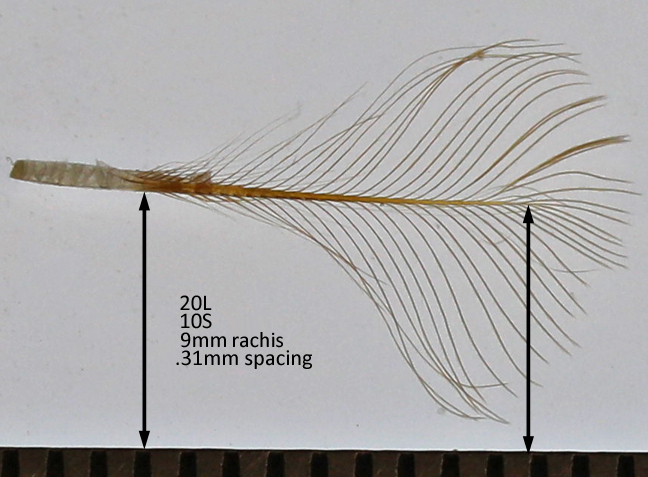
CHIC barb spacing
Barb angle is measured using the general direction of the barb. The angle will typically be smaller where the barb meets the rachis. Pictures are placed on top of an on-line tool.
https://www.ginifab.com/feeds/angle_measurement/
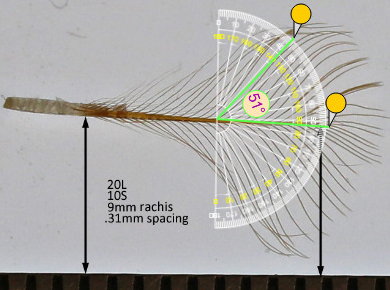
Chicken CHIC barb angle
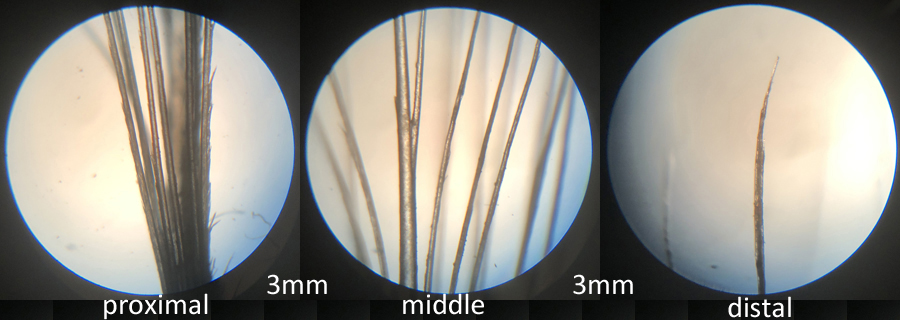
Morphology of the vane, barbs and barbules are noted. Three sets of microscopic views are made of a feather at three magnifications. The first view is of the aftershaft just above the calamus. The magnifications are 50X, 150X, and 300X.

proximal of vane
The second view is the middle of the vane to the outside edge. This look provides observations on barbule growth. Magnification is at 50X. Note that barbule growth increases towards the distal end of the barbs.
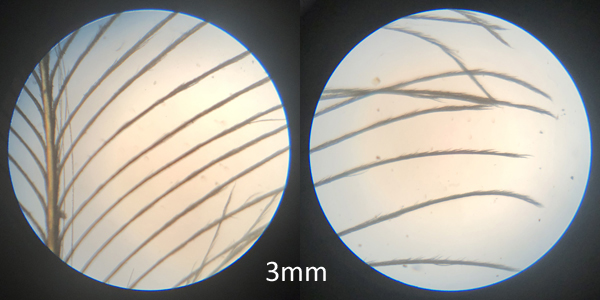
middle of vane
This third view is of the crown of the feather. Note the significant barbule growth. The magnifications are 50X, 150X, and 300X.
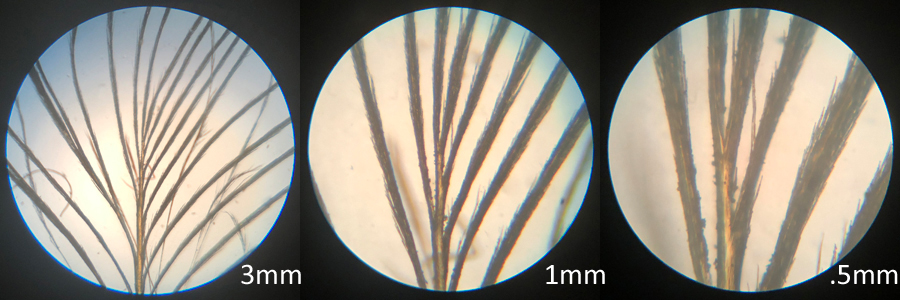
end of vane
Equipment Used
Macro Photography
Lens — Canon Macro Lens EF 100mm F2.8
Remote Switch — SMDV wired remote shutter release
Tripod head — Benrousa 3-way geared head

camera gear
Lightpad

AGPtEK light pad
Microscopes
Edmund Scientific 300X microscope and reflected light illuminator.

Edmond microscope

WiFi microscope
Micro Photography

iPhone 15 on Edmund microscope
Dissecting Kit
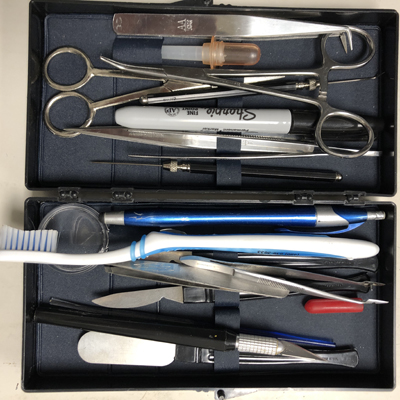
dissecting kit
Magnifying work light
Indispensable for close up work. This link is for the latest model.

magnifying work light
Digital caliper

caliper
Ethics Statement
No birds were killed for this study. Some birds, based on condition, were set out for neighborhood owls. Provenance of the birds is maintained and reported annually (while license is valid) to Arizona Game and Fish Department and U.S. Fish and Wildlife Service. All feathers will be donated to an educational institution upon completion of the study.
Acknowledgements
Ted Bodner — BS Biology, MS Pathology, professional bird guide, professional photographer, mentor for 14 years
Laurie Nessel — editor for the Maricopa Audubon Society’s quarterly magazine Cactus Wren-dition and monthly newsletter — published articles, requested support for birds from members.
https://www.maricopaaudubon.org/
Wild at Heart Raptor Rehab facility — supplied 11 raptors and Greater Roadrunner
https://www.wildatheartraptors.org
Damon “Rooster” Cogburn — generously supplied an ostrich, if you live in Arizona take the kids to this wonderful ranch
Elizabeth Farquhar — editor of Desert Rivers Audubon Society’s quarterly magazine and newsletter. Published request for birds.
References
1 Rohwer, VG, Rohwer, S, Kane, L. (2021) Filoplume morphology covaries with their companion primary suggesting that they are feather-specific sensors. Ornithology 138, 2021, pp. 1–11
2 Clark, Jr., GA and de Cruz, JB. (1989) Functional interpretation of protruding filoplumes in oscines. The Condor 91:962-965
3 Devokaitis, Marc (2020) The Most Mysterious Feather: Filoplumes. Cornell “https://www.allaboutbirds.org/news/the-most-mysterious-feather-filoplumes/#”
4 Seneviratne, S.S. and Jones, I.L. (2008) Mechanosensory function for facial ornamentation in the whiskered auklet, a crevice-dwelling seabird. Behavioral Ecology · July 2008, 784-790
5 Conover, R. & Miller, E. (1980) Rictal bristle function in willow flycatcher. Condor, 82, 469–471
6 Delaunay, MG, Brassey, C, Larsen, C, Lloyd, H and Grant, RA. (2022) The evolutionary origin of avian facial bristles and the likely role of rictal bristles in feeding ecology. Scientific Reports (2022) 12:21108
7 Delaunay, M.G., Larsen, C., Lloyd, H., Sullivan, M., and Grant, R.A. (2020). Anatomy of avian rictal bristles in aprimulgiformes reveals reduced tactile function in open-habitat, partially diurnal foraging species. Journal of Anatomy, 237:355–366
8 Delaunay, M.G., Charter, M. & Grant, R.A. (2022) Anatomy of bristles on the nares and rictus of western barn owls (Tyto Alba). Journal of Anatomy, 241, 527–534
9 Lederer, R.J. (1972) The role of avian rictal bristles. The Wilson Bulletin, 84(2), 193–197
10 Conover, R. & Miller, E. (1980) Rictal bristle function in willow flycatcher. Condor 82, 469–471
11 Kane SA, Van Beveren D, Dakin R (2018) Biomechanics of the peafowl’s crest reveals frequencies tuned to social displays. PLoS ONE 13(11): e0207247.
12 Koch UR and Wagner H. (2002) Morphometry of Auricular Feathers of Barn Owls. European Journal of Morphology, Vol. 40, No. 1, pp. 15–21
13 Novak, S. (2024) Beasts with Brains. Discover, January 2024, 44-45.
14 West, G. Scaling Biology with Geoffrey West. May 2022, Thinking Tools Podcast #25.
Ancillary Topics
blank
